zolmitriptan
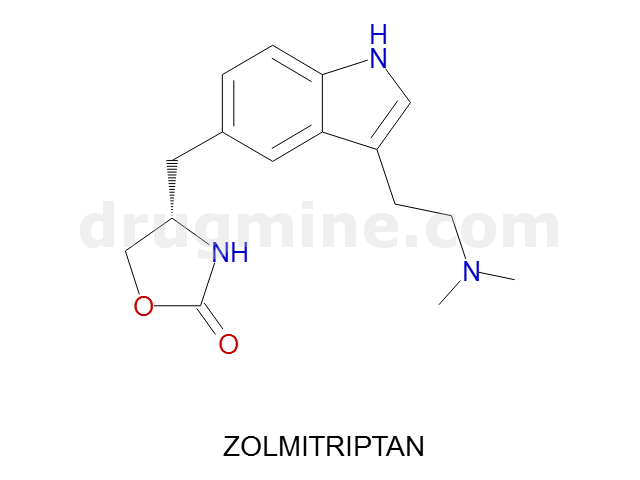
Name: ZOLMITRIPTAN
ID :
MW: 287
Number of atoms: 21
Molecular_Formula: C16H21N3O2
Alogp: 2.457
Indication class : Antimigraine
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
zolmitriptan containing products summary
There are in total 26 different products containing the active ingredient zolmitriptan. From the 26 drug products, zero have been discontinued.Product id = 29866
Application Number = 20768
Date of Application = 25,, Nov, 1997
RX/OTC/DISCN = RX
Tradename = ZOMIG
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IPR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29867
Application Number = 20768
Date of Application = 25,, Nov, 1997
RX/OTC/DISCN = RX
Tradename = ZOMIG
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IPR
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 17119
Application Number = 21231
Date of Application = 13,, Feb, 2001
RX/OTC/DISCN = RX
Tradename = ZOMIG-ZMT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 17120
Application Number = 21231
Date of Application = 17,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ZOMIG-ZMT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 14411
Application Number = 21450
Date of Application = 16,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = ZOMIG
Route/format = NASAL / SPRAY
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 003
Tecode =
Rld = No
Strength = 2.5MG/SPRAY
----
Product id = 14412
Application Number = 21450
Date of Application = 30,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = ZOMIG
Route/format = NASAL / SPRAY
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 004
Tecode =
Rld = Yes
Strength = 5MG/SPRAY
----
Product id = 29817
Application Number = 90861
Date of Application = 4,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29818
Application Number = 90861
Date of Application = 4,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29807
Application Number = 201779
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29808
Application Number = 201779
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29805
Application Number = 202078
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29806
Application Number = 202078
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29811
Application Number = 202279
Date of Application = 20,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29812
Application Number = 202279
Date of Application = 20,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 17113
Application Number = 202476
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 17114
Application Number = 202476
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 17115
Application Number = 202560
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 17116
Application Number = 202560
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 17117
Application Number = 202890
Date of Application = 15,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 17118
Application Number = 202890
Date of Application = 15,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29813
Application Number = 203186
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29814
Application Number = 203186
Date of Application = 14,, May, 2013
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29815
Application Number = 203476
Date of Application = 13,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29816
Application Number = 203476
Date of Application = 13,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29809
Application Number = 204284
Date of Application = 9,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29810
Application Number = 204284
Date of Application = 9,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = ZOLMITRIPTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----