triamcinolone-acetonide
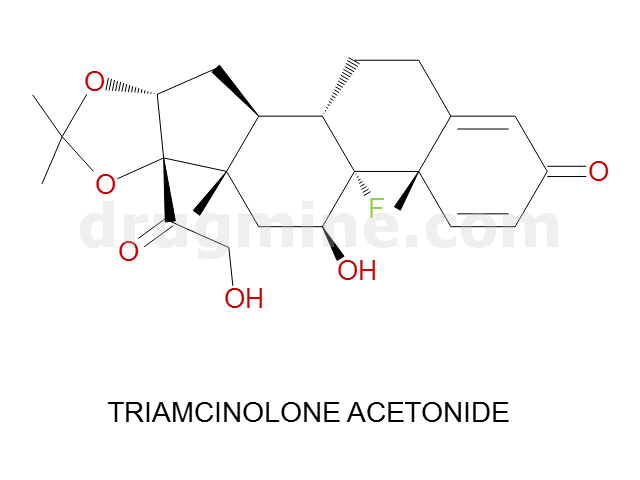
Name: TRIAMCINOLONE ACETONIDE
ID :
MW: 434
Number of atoms: 31
Molecular_Formula: C24H31FO6
Alogp: 1.157
Indication class : Glucocorticoid
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
triamcinolone acetonide containing products summary
There are in total 153 different products containing the active ingredient triamcinolone acetonide. From the 153 drug products, 116 have been discontinued.Product id = 12517
Application Number = 11600
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12516
Application Number = 11600
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 003
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4275
Application Number = 11601
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 003
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4276
Application Number = 11601
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 006
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12222
Application Number = 11602
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12220
Application Number = 11602
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 003
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 8605
Application Number = 12041
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KENALOG-10
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 12623
Application Number = 12097
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG IN ORABASE
Route/format = DENTAL / PASTE
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 14422
Application Number = 12104
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KENALOG
Route/format = TOPICAL / SPRAY
Application Type = N
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.147MG/GM
----
Product id = 8606
Application Number = 14901
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KENALOG-40
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = Yes
Strength = 40MG/ML
----
Product id = 36
Application Number = 18117
Date of Application = 23,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = AZMACORT
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG/INH
----
Product id = 11199
Application Number = 19503
Date of Application = 16,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PARNELL
ProductNo = 001
Tecode =
Rld = No
Strength = 3MG/ML
----
Product id = 84
Application Number = 19798
Date of Application = 11,, Jul, 1991
RX/OTC/DISCN = DISCN
Tradename = NASACORT
Route/format = NASAL / AEROSOL, METERED
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 0.055MG/INH
----
Product id = 14330
Application Number = 20120
Date of Application = 4,, Feb, 2000
RX/OTC/DISCN = DISCN
Tradename = ALLERNAZE
Route/format = NASAL / SPRAY, METERED
Application Type = N
Applicant Name = LUPIN ATLANTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05MG/SPRAY
----
Product id = 14382
Application Number = 20468
Date of Application = 11,, Oct, 2013
RX/OTC/DISCN = OTC
Tradename = NASACORT ALLERGY 24 HOUR
Route/format = NASAL / SPRAY, METERED
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = Yes
Strength = 0.055MG/SPRAY
----
Product id = 14383
Application Number = 20784
Date of Application = 7,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = NASACORT HFA
Route/format = NASAL / SPRAY, METERED
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 0.055MG/SPRAY
----
Product id = 11738
Application Number = 22048
Date of Application = 29,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = TRIESENCE
Route/format = INTRAVITREAL / INJECTABLE
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode =
Rld = Yes
Strength = 40MG/ML (40MG/ML)
----
Product id = 11504
Application Number = 22220
Date of Application = 16,, Jun, 2008
RX/OTC/DISCN = DISCN
Tradename = TRIVARIS
Route/format = INTRA-ARTICULAR, INTRAMUSCULAR, INTRAVITREAL / INJECTABLE
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode =
Rld = No
Strength = 8MG/0.1ML (8MG/0.1ML)
----
Product id = 12601
Application Number = 40037
Date of Application = 30,, Sep, 1994
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 4430
Application Number = 40038
Date of Application = 26,, Oct, 1994
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4432
Application Number = 40039
Date of Application = 26,, Nov, 1997
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 12599
Application Number = 40040
Date of Application = 30,, Sep, 1994
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12600
Application Number = 40374
Date of Application = 5,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12603
Application Number = 40386
Date of Application = 5,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12266
Application Number = 40467
Date of Application = 21,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 12267
Application Number = 40467
Date of Application = 21,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 002
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 4438
Application Number = 40671
Date of Application = 9,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4439
Application Number = 40671
Date of Application = 9,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = VINTAGE
ProductNo = 002
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 12271
Application Number = 40672
Date of Application = 13,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = VINTAGE
ProductNo = 002
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 12625
Application Number = 40771
Date of Application = 1,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = DENTAL / PASTE
Application Type = A
Applicant Name = LYNE
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 12542
Application Number = 60572
Date of Application = 28,, Jun, 1985
RX/OTC/DISCN = DISCN
Tradename = MYCOLOG-II
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4320
Application Number = 60576
Date of Application = 1,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = MYCOLOG-II
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 002
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4319
Application Number = 61954
Date of Application = 20,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = MYCO-TRIACET II
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12541
Application Number = 62045
Date of Application = 26,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = MYCO-TRIACET II
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4354
Application Number = 62186
Date of Application = 6,, Jun, 1985
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 002
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12565
Application Number = 62280
Date of Application = 10,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 002
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4356
Application Number = 62347
Date of Application = 30,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4357
Application Number = 62364
Date of Application = 22,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4323
Application Number = 62367
Date of Application = 28,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = MYKACET
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4337
Application Number = 62595
Date of Application = 21,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = NEOMYCIN SULFATE-TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.1%
----
Product id = 4358
Application Number = 62596
Date of Application = 8,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN-TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4326
Application Number = 62597
Date of Application = 8,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = MYTREX F
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4325
Application Number = 62598
Date of Application = 21,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = MYTREX A
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.1%
----
Product id = 4353
Application Number = 62599
Date of Application = 8,, Oct, 1985
RX/OTC/DISCN = RX
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4336
Application Number = 62600
Date of Application = 21,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = NEOMYCIN SULFATE-TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.1%
----
Product id = 12547
Application Number = 62601
Date of Application = 9,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = MYTREX F
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12564
Application Number = 62602
Date of Application = 9,, Oct, 1985
RX/OTC/DISCN = RX
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12568
Application Number = 62603
Date of Application = 9,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN-TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4321
Application Number = 62606
Date of Application = 15,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = MYCOLOG-II
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12553
Application Number = 62607
Date of Application = 23,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = NEOMYCIN SULFATE-TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.1%
----
Product id = 12552
Application Number = 62608
Date of Application = 23,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = NEOMYCIN SULFATE-TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.1%
----
Product id = 12546
Application Number = 62609
Date of Application = 23,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = MYTREX A
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.1%
----
Product id = 12566
Application Number = 62656
Date of Application = 30,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4355
Application Number = 62657
Date of Application = 30,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12544
Application Number = 62733
Date of Application = 9,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = MYKACET
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 4352
Application Number = 63010
Date of Application = 20,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12567
Application Number = 63305
Date of Application = 29,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = NYSTATIN AND TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 100,000 UNITS/GM;0.1%
----
Product id = 12626
Application Number = 70730
Date of Application = 1,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = DENTAL / PASTE
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.1%
----
Product id = 12624
Application Number = 71383
Date of Application = 6,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = ORALONE
Route/format = DENTAL / PASTE
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 14400
Application Number = 78104
Date of Application = 30,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = NASAL / SPRAY, METERED
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.055MG/SPRAY
----
Product id = 12398
Application Number = 80745
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12401
Application Number = 80745
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 003
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12399
Application Number = 80750
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 003
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12397
Application Number = 80750
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 004
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4043
Application Number = 83015
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4048
Application Number = 83015
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 003
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4042
Application Number = 83016
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 004
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4046
Application Number = 83016
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 005
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4041
Application Number = 83017
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 003
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4044
Application Number = 83017
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 004
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 5367
Application Number = 83380
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ARISTOGEL
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4277
Application Number = 83943
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12518
Application Number = 83944
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12219
Application Number = 84343
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12221
Application Number = 84343
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4406
Application Number = 84908
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIACET
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4407
Application Number = 84908
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIACET
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4408
Application Number = 84908
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIACET
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4185
Application Number = 85539
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FLUTEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4186
Application Number = 85539
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FLUTEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4187
Application Number = 85539
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = FLUTEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12586
Application Number = 85691
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 12588
Application Number = 85691
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 002
Tecode = AT
Rld = No
Strength = 0.5%
----
Product id = 12587
Application Number = 85691
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 003
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 4413
Application Number = 85692
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.025%
----
Product id = 4415
Application Number = 85692
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 002
Tecode = AT
Rld = Yes
Strength = 0.5%
----
Product id = 4414
Application Number = 85692
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 003
Tecode = AT
Rld = Yes
Strength = 0.1%
----
Product id = 11202
Application Number = 85825
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/ML
----
Product id = 4278
Application Number = 86240
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KENALOG-H
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4434
Application Number = 86275
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4433
Application Number = 86276
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4431
Application Number = 86277
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4423
Application Number = 86413
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.5%
----
Product id = 4422
Application Number = 86414
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 4421
Application Number = 86415
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4409
Application Number = 87113
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIACORT
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12264
Application Number = 87191
Date of Application = 8,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12265
Application Number = 87192
Date of Application = 8,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12594
Application Number = 87356
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.025%
----
Product id = 12595
Application Number = 87357
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.1%
----
Product id = 12467
Application Number = 87375
Date of Application = 1,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = FLUTEX
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12469
Application Number = 87376
Date of Application = 1,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = FLUTEX
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12468
Application Number = 87377
Date of Application = 1,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = FLUTEX
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12596
Application Number = 87385
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.5%
----
Product id = 4442
Application Number = 87428
Date of Application = 1,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIATEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4441
Application Number = 87429
Date of Application = 1,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIATEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4440
Application Number = 87430
Date of Application = 1,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIATEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4411
Application Number = 87797
Date of Application = 7,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4410
Application Number = 87798
Date of Application = 4,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12584
Application Number = 87799
Date of Application = 7,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12602
Application Number = 87902
Date of Application = 27,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4428
Application Number = 87912
Date of Application = 10,, Aug, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4427
Application Number = 87921
Date of Application = 10,, Aug, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4429
Application Number = 87922
Date of Application = 10,, Aug, 1982
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4412
Application Number = 87932
Date of Application = 9,, May, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = AMBIX
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4424
Application Number = 87990
Date of Application = 7,, Jul, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4425
Application Number = 87991
Date of Application = 7,, Jul, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4426
Application Number = 87992
Date of Application = 7,, Jul, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4443
Application Number = 88042
Date of Application = 19,, Mar, 1984
RX/OTC/DISCN = RX
Tradename = TRIDERM
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = CROWN LABS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.1%
----
Product id = 12591
Application Number = 88090
Date of Application = 1,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12592
Application Number = 88091
Date of Application = 1,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12593
Application Number = 88092
Date of Application = 1,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4418
Application Number = 88094
Date of Application = 1,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4419
Application Number = 88095
Date of Application = 1,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4420
Application Number = 88096
Date of Application = 1,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4444
Application Number = 88196
Date of Application = 25,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = TRYMEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4445
Application Number = 88197
Date of Application = 25,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = TRYMEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4446
Application Number = 88198
Date of Application = 25,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = TRYMEX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12273
Application Number = 88450
Date of Application = 1,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = WOCKHARDT EU OPERATN
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.025%
----
Product id = 12272
Application Number = 88451
Date of Application = 3,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.1%
----
Product id = 12598
Application Number = 88690
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12606
Application Number = 88691
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = TRYMEX
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12597
Application Number = 88692
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12605
Application Number = 88693
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = TRYMEX
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12400
Application Number = 88780
Date of Application = 1,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12402
Application Number = 88781
Date of Application = 5,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 4045
Application Number = 88818
Date of Application = 16,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4047
Application Number = 88819
Date of Application = 16,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4049
Application Number = 88820
Date of Application = 16,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = ARISTOCORT A
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ASTELLAS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12268
Application Number = 89129
Date of Application = 14,, Aug, 1986
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4435
Application Number = 89274
Date of Application = 21,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TOPIDERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4436
Application Number = 89275
Date of Application = 21,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TOPIDERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4437
Application Number = 89276
Date of Application = 21,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TOPIDERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 12604
Application Number = 89595
Date of Application = 23,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE IN ABSORBASE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = CAROLINA MEDCL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.05%
----
Product id = 12589
Application Number = 89795
Date of Application = 23,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12590
Application Number = 89796
Date of Application = 23,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 4416
Application Number = 89797
Date of Application = 31,, May, 1991
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4417
Application Number = 89798
Date of Application = 31,, May, 1991
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%
----
Product id = 12585
Application Number = 89913
Date of Application = 23,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%
----
Product id = 11201
Application Number = 90164
Date of Application = 1,, Jun, 2009
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ CANADA INC
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/ML
----
Product id = 11200
Application Number = 90166
Date of Application = 27,, May, 2009
RX/OTC/DISCN = DISCN
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 12269
Application Number = 202374
Date of Application = 8,, May, 2013
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = VERSAPHARM INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 12270
Application Number = 202374
Date of Application = 8,, May, 2013
RX/OTC/DISCN = RX
Tradename = TRIAMCINOLONE ACETONIDE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = VERSAPHARM INC
ProductNo = 002
Tecode = AT
Rld = No
Strength = 0.1%
----