trazodone-hydrochloride
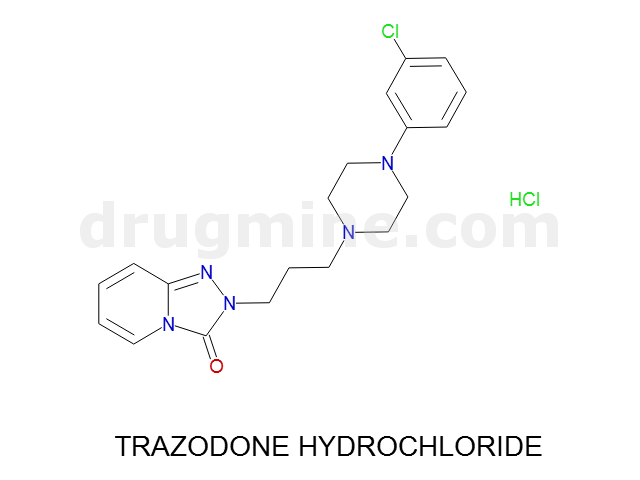
Name: TRAZODONE HYDROCHLORIDE
ID :
MW: 372
Number of atoms: 26
Molecular_Formula: C19H22ClN5O
Alogp: 2.418
Indication class : Antidepressant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
trazodone hydrochloride containing products summary
There are in total 43 different products containing the active ingredient trazodone hydrochloride. From the 43 drug products, 19 have been discontinued.Product id = 20210
Application Number = 18207
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DESYREL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20211
Application Number = 18207
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DESYREL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 20212
Application Number = 18207
Date of Application = 25,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = DESYREL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 20213
Application Number = 18207
Date of Application = 7,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = DESYREL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 004
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16388
Application Number = 22411
Date of Application = 2,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = OLEPTRO
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANGELINI PHARMA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 150MG
----
Product id = 16389
Application Number = 22411
Date of Application = 2,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = OLEPTRO
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANGELINI PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 29088
Application Number = 70491
Date of Application = 29,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29089
Application Number = 70492
Date of Application = 29,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29092
Application Number = 70857
Date of Application = 10,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29094
Application Number = 70858
Date of Application = 10,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29078
Application Number = 70921
Date of Application = 1,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29105
Application Number = 70942
Date of Application = 1,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = TRIALODINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29093
Application Number = 71112
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29095
Application Number = 71113
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29062
Application Number = 71139
Date of Application = 29,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29063
Application Number = 71140
Date of Application = 29,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29065
Application Number = 71196
Date of Application = 25,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 29066
Application Number = 71196
Date of Application = 26,, Apr, 1999
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 29067
Application Number = 71196
Date of Application = 26,, Apr, 1999
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 29064
Application Number = 71258
Date of Application = 25,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29071
Application Number = 71405
Date of Application = 27,, Feb, 1991
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29072
Application Number = 71406
Date of Application = 27,, Feb, 1991
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29061
Application Number = 71514
Date of Application = 18,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALVOGEN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 29082
Application Number = 71523
Date of Application = 11,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29083
Application Number = 71524
Date of Application = 11,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 29077
Application Number = 71525
Date of Application = 9,, Mar, 1988
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 29060
Application Number = 71636
Date of Application = 18,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALVOGEN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29090
Application Number = 72192
Date of Application = 2,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29091
Application Number = 72193
Date of Application = 2,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 29080
Application Number = 72483
Date of Application = 30,, Apr, 1990
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 29079
Application Number = 72484
Date of Application = 30,, Apr, 1990
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29069
Application Number = 73137
Date of Application = 24,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 29068
Application Number = 73137
Date of Application = 24,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29070
Application Number = 73137
Date of Application = 22,, Dec, 1995
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 29081
Application Number = 74357
Date of Application = 30,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 29073
Application Number = 90514
Date of Application = 2,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29074
Application Number = 90514
Date of Application = 2,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 29075
Application Number = 90514
Date of Application = 2,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 29076
Application Number = 90514
Date of Application = 2,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 29084
Application Number = 202180
Date of Application = 27,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29085
Application Number = 202180
Date of Application = 27,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 29086
Application Number = 202180
Date of Application = 27,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 29087
Application Number = 202180
Date of Application = 27,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = TRAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 300MG
----