tranexamic-acid
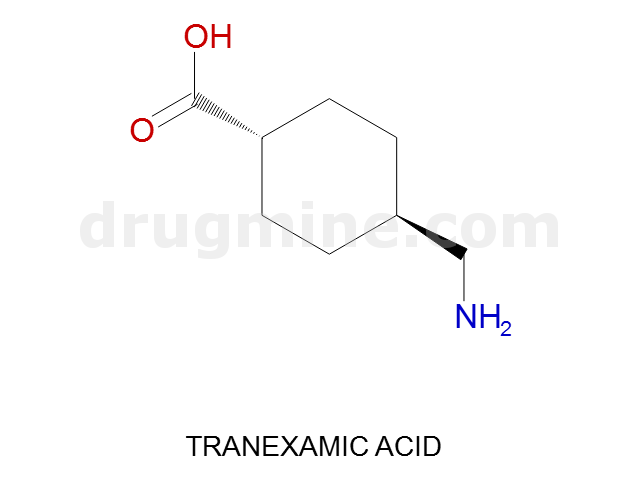


Name: TRANEXAMIC ACID
ID :
MW: 157
Number of atoms: 11
Molecular_Formula: C8H15NO2
Alogp: -1.753
Indication class : Hemostatic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
tranexamic acid containing products summary
There are in total 12 different products containing the active ingredient tranexamic acid. From the 12 drug products, 2 have been discontinued.Product id = 20081
Application Number = 19280
Date of Application = 30,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = CYKLOKAPRON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 6908
Application Number = 19281
Date of Application = 30,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = CYKLOKAPRON
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 100MG/ML
----
Product id = 24104
Application Number = 22430
Date of Application = 13,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = LYSTEDA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = FERRING PHARMS AS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 650MG
----
Product id = 11158
Application Number = 91596
Date of Application = 2,, Mar, 2012
RX/OTC/DISCN = DISCN
Tradename = TRANEXAMIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 11160
Application Number = 91657
Date of Application = 3,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----
Product id = 11162
Application Number = 201580
Date of Application = 14,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = X-GEN PHARMS INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----
Product id = 11159
Application Number = 201885
Date of Application = 10,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----
Product id = 29050
Application Number = 202093
Date of Application = 27,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 650MG
----
Product id = 29051
Application Number = 202286
Date of Application = 27,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 650MG
----
Product id = 11161
Application Number = 202373
Date of Application = 17,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = VERSAPHARM INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----
Product id = 11156
Application Number = 202436
Date of Application = 11,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACIC FINE CHEMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----
Product id = 11157
Application Number = 203521
Date of Application = 12,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TRANEXAMIC ACID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----