trandolapril
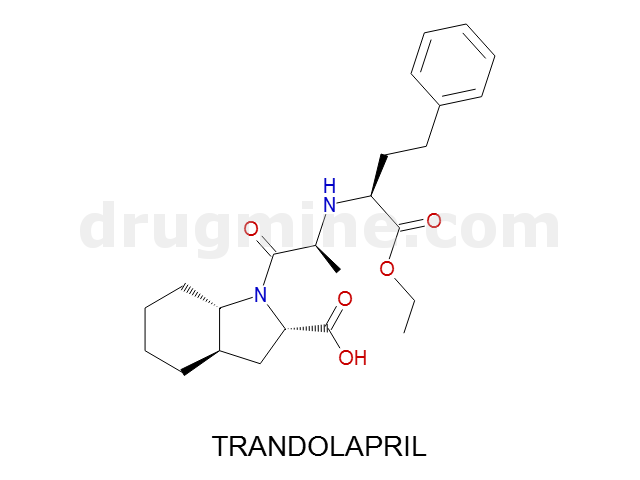

Name: TRANDOLAPRIL
ID :
MW: 431
Number of atoms: 31
Molecular_Formula: C24H34N2O5
Alogp: 0.764
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
trandolapril containing products summary
There are in total 41 different products containing the active ingredient trandolapril. From the 41 drug products, 9 have been discontinued.Product id = 24123
Application Number = 20528
Date of Application = 26,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = MAVIK
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 24124
Application Number = 20528
Date of Application = 26,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = MAVIK
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 24125
Application Number = 20528
Date of Application = 26,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = MAVIK
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 4MG
----
Product id = 16659
Application Number = 20591
Date of Application = 22,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = TARKA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG;180MG
----
Product id = 16661
Application Number = 20591
Date of Application = 22,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = TARKA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 4MG;240MG
----
Product id = 16658
Application Number = 20591
Date of Application = 22,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = TARKA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG;240MG
----
Product id = 16660
Application Number = 20591
Date of Application = 22,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = TARKA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG;240MG
----
Product id = 29026
Application Number = 77256
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 29027
Application Number = 77256
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG
----
Product id = 29028
Application Number = 77256
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG
----
Product id = 29024
Application Number = 77307
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 29023
Application Number = 77307
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 002
Tecode =
Rld = No
Strength = 1MG
----
Product id = 29025
Application Number = 77307
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG
----
Product id = 29044
Application Number = 77489
Date of Application = 12,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 29045
Application Number = 77489
Date of Application = 12,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 29046
Application Number = 77489
Date of Application = 12,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 29038
Application Number = 77522
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 29039
Application Number = 77522
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 29040
Application Number = 77522
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 29047
Application Number = 77805
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 29048
Application Number = 77805
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 29049
Application Number = 77805
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 29035
Application Number = 78320
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 29036
Application Number = 78320
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 29037
Application Number = 78320
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 29041
Application Number = 78346
Date of Application = 28,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 29042
Application Number = 78346
Date of Application = 28,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 29043
Application Number = 78346
Date of Application = 28,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 29020
Application Number = 78438
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 29021
Application Number = 78438
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 29022
Application Number = 78438
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 29029
Application Number = 78493
Date of Application = 25,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 29030
Application Number = 78493
Date of Application = 25,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG
----
Product id = 29031
Application Number = 78493
Date of Application = 25,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG
----
Product id = 29033
Application Number = 78508
Date of Application = 18,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EPIC PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 29034
Application Number = 78508
Date of Application = 18,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EPIC PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 29032
Application Number = 78508
Date of Application = 18,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EPIC PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 16725
Application Number = 79135
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG;180MG
----
Product id = 16726
Application Number = 79135
Date of Application = 26,, May, 2010
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG;240MG
----
Product id = 16727
Application Number = 79135
Date of Application = 5,, May, 2010
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG;240MG
----
Product id = 16724
Application Number = 79135
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = TRANDOLAPRIL AND VERAPAMIL HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 1MG;240MG
----