tobramycin
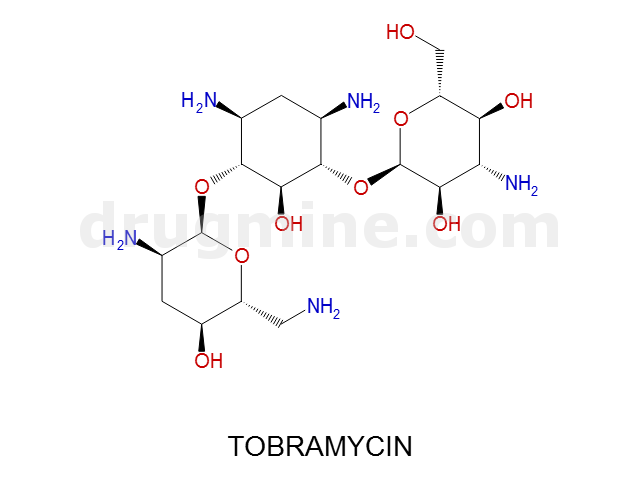
Name: TOBRAMYCIN
ID :
MW: 468
Number of atoms: 32
Molecular_Formula: C18H37N5O9
Alogp: -6.86
Indication class : Antibacterial
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
tobramycin containing products summary
There are in total 20 different products containing the active ingredient tobramycin. From the 20 drug products, 3 have been discontinued.Product id = 13172
Application Number = 50541
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TOBREX
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = FALCON PHARMS
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.3%
----
Product id = 12376
Application Number = 50555
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TOBREX
Route/format = OPHTHALMIC / OINTMENT
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.3%
----
Product id = 14581
Application Number = 50592
Date of Application = 18,, Aug, 1988
RX/OTC/DISCN = RX
Tradename = TOBRADEX
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.1%;0.3%
----
Product id = 12375
Application Number = 50616
Date of Application = 28,, Sep, 1988
RX/OTC/DISCN = RX
Tradename = TOBRADEX
Route/format = OPHTHALMIC / OINTMENT
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.1%;0.3%
----
Product id = 14584
Application Number = 50628
Date of Application = 21,, Jul, 1989
RX/OTC/DISCN = DISCN
Tradename = TOBRASONE
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1%;0.3%
----
Product id = 13404
Application Number = 50753
Date of Application = 22,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = TOBI
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = NOVARTIS PHARMS
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = 300MG/5ML
----
Product id = 14586
Application Number = 50804
Date of Application = 14,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = ZYLET
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.5%;0.3%
----
Product id = 14582
Application Number = 50818
Date of Application = 13,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = TOBRADEX ST
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.05%;0.3%
----
Product id = 13171
Application Number = 62535
Date of Application = 13,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = TOBREX
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.3%
----
Product id = 13167
Application Number = 63176
Date of Application = 25,, May, 1994
RX/OTC/DISCN = DISCN
Tradename = TOBRAMYCIN
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 0.3%
----
Product id = 13169
Application Number = 64052
Date of Application = 29,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = TOBRAMYCIN
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.3%
----
Product id = 12848
Application Number = 64096
Date of Application = 31,, Jan, 1996
RX/OTC/DISCN = RX
Tradename = AKTOB
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.3%
----
Product id = 14583
Application Number = 64134
Date of Application = 27,, Oct, 1999
RX/OTC/DISCN = RX
Tradename = TOBRAMYCIN AND DEXAMETHASONE
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1%;0.3%
----
Product id = 13170
Application Number = 65026
Date of Application = 11,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = TOBRAMYCIN
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = FERA PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.3%
----
Product id = 13168
Application Number = 65087
Date of Application = 25,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = TOBRAMYCIN
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.3%
----
Product id = 13406
Application Number = 91589
Date of Application = 10,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = TOBRAMYCIN
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AN
Rld = No
Strength = 300MG/5ML
----
Product id = 13405
Application Number = 201422
Date of Application = 28,, May, 2014
RX/OTC/DISCN = RX
Tradename = TOBRAMYCIN
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AN
Rld = No
Strength = 300MG/5ML
----
Product id = 12693
Application Number = 201688
Date of Application = 22,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = TOBI PODHALER
Route/format = INHALATION / POWDER
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 28MG
----
Product id = 13296
Application Number = 201820
Date of Application = 12,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = BETHKIS
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = CHIESI USA INC
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG/4ML
----
Product id = 13369
Application Number = 205433
Date of Application = 2,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = KITABIS PAK
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = PULMOFLOW INC
ProductNo = 001
Tecode = AN
Rld = No
Strength = 300MG/5ML
----