tizanidine-hydrochloride
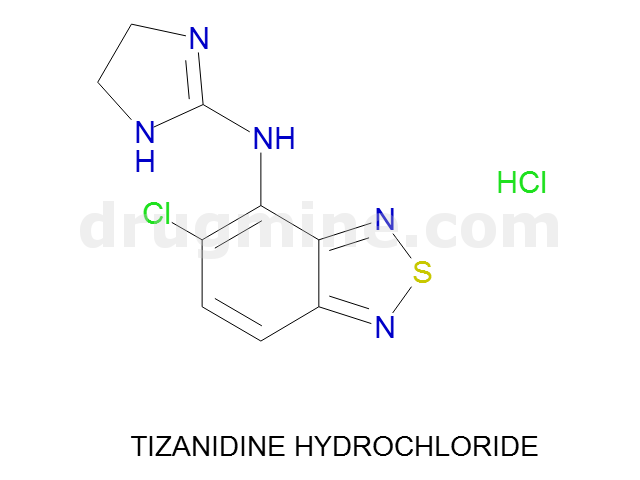

Name: TIZANIDINE HYDROCHLORIDE
ID :
MW: 254
Number of atoms: 16
Molecular_Formula: C9H8ClN5S
Alogp: 1.817
Indication class : Antispasmodic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
tizanidine hydrochloride containing products summary
There are in total 37 different products containing the active ingredient tizanidine hydrochloride. From the 37 drug products, 9 have been discontinued.Product id = 29756
Application Number = 20397
Date of Application = 27,, Nov, 1996
RX/OTC/DISCN = RX
Tradename = ZANAFLEX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ACORDA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 4MG BASE
----
Product id = 29755
Application Number = 20397
Date of Application = 4,, Feb, 2000
RX/OTC/DISCN = DISCN
Tradename = ZANAFLEX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ACORDA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 3794
Application Number = 21447
Date of Application = 29,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = ZANAFLEX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ACORDA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 3795
Application Number = 21447
Date of Application = 29,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = ZANAFLEX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ACORDA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 3796
Application Number = 21447
Date of Application = 29,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = ZANAFLEX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ACORDA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 6MG BASE
----
Product id = 28766
Application Number = 76280
Date of Application = 26,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28767
Application Number = 76280
Date of Application = 27,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28764
Application Number = 76281
Date of Application = 20,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28765
Application Number = 76281
Date of Application = 20,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28762
Application Number = 76282
Date of Application = 16,, Dec, 2003
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28763
Application Number = 76282
Date of Application = 16,, Dec, 2003
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28748
Application Number = 76283
Date of Application = 12,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28749
Application Number = 76283
Date of Application = 12,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28770
Application Number = 76284
Date of Application = 3,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28771
Application Number = 76284
Date of Application = 3,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28756
Application Number = 76286
Date of Application = 3,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28757
Application Number = 76286
Date of Application = 3,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28758
Application Number = 76321
Date of Application = 30,, Sep, 2004
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28759
Application Number = 76321
Date of Application = 30,, Sep, 2004
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28754
Application Number = 76347
Date of Application = 11,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28755
Application Number = 76347
Date of Application = 11,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28760
Application Number = 76354
Date of Application = 28,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28761
Application Number = 76354
Date of Application = 28,, Mar, 2003
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28752
Application Number = 76371
Date of Application = 9,, Apr, 2003
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28753
Application Number = 76371
Date of Application = 9,, Apr, 2003
RX/OTC/DISCN = DISCN
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28768
Application Number = 76416
Date of Application = 29,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28769
Application Number = 76416
Date of Application = 29,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 28750
Application Number = 76533
Date of Application = 16,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28751
Application Number = 76533
Date of Application = 16,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 3594
Application Number = 78868
Date of Application = 3,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 3595
Application Number = 78868
Date of Application = 3,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 3596
Application Number = 78868
Date of Application = 3,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----
Product id = 28772
Application Number = 91283
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 28773
Application Number = 91283
Date of Application = 28,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 3597
Application Number = 91502
Date of Application = 9,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 3598
Application Number = 91502
Date of Application = 9,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 3599
Application Number = 91502
Date of Application = 9,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = TIZANIDINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 6MG BASE
----