theophylline
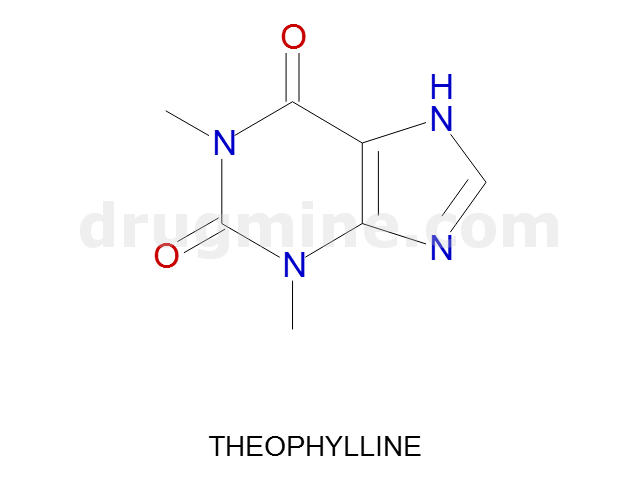

Name: THEOPHYLLINE
ID :
MW: 180
Number of atoms: 13
Molecular_Formula: C7H8N4O2
Alogp: -0.306
Indication class : Bronchodilator
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
theophylline containing products summary
There are in total 159 different products containing the active ingredient theophylline. From the 159 drug products, 128 have been discontinued.Product id = 7217
Application Number = 8869
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DICURIN PROCAINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML;50MG/ML
----
Product id = 11040
Application Number = 18649
Date of Application = 26,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/100ML
----
Product id = 11041
Application Number = 18649
Date of Application = 26,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 80MG/100ML
----
Product id = 11042
Application Number = 18649
Date of Application = 26,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = 160MG/100ML
----
Product id = 11043
Application Number = 18649
Date of Application = 26,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 11045
Application Number = 18649
Date of Application = 26,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 005
Tecode =
Rld = No
Strength = 400MG/100ML
----
Product id = 11044
Application Number = 18649
Date of Application = 13,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 006
Tecode =
Rld = No
Strength = 320MG/100ML
----
Product id = 11039
Application Number = 18649
Date of Application = 26,, Jul, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 007
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 11027
Application Number = 19083
Date of Application = 7,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.04% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/100ML
----
Product id = 11029
Application Number = 19083
Date of Application = 7,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.08% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = No
Strength = 80MG/100ML
----
Product id = 11031
Application Number = 19083
Date of Application = 7,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.16% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 003
Tecode =
Rld = No
Strength = 160MG/100ML
----
Product id = 11047
Application Number = 19211
Date of Application = 14,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 40MG/100ML
----
Product id = 11048
Application Number = 19211
Date of Application = 14,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode =
Rld = No
Strength = 80MG/100ML
----
Product id = 11049
Application Number = 19211
Date of Application = 14,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 160MG/100ML
----
Product id = 11050
Application Number = 19211
Date of Application = 14,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 11052
Application Number = 19211
Date of Application = 14,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 005
Tecode =
Rld = No
Strength = 400MG/100ML
----
Product id = 11051
Application Number = 19211
Date of Application = 20,, Jan, 1988
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 006
Tecode = AP
Rld = Yes
Strength = 320MG/100ML
----
Product id = 11046
Application Number = 19211
Date of Application = 14,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 007
Tecode = AP
Rld = Yes
Strength = 4MG/ML
----
Product id = 11033
Application Number = 19212
Date of Application = 7,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.2% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 11037
Application Number = 19212
Date of Application = 7,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.4% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG/100ML
----
Product id = 11036
Application Number = 19212
Date of Application = 7,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.4% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG/ML
----
Product id = 11028
Application Number = 19826
Date of Application = 14,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE 0.04% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 40MG/100ML
----
Product id = 11030
Application Number = 19826
Date of Application = 14,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE 0.08% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 80MG/100ML
----
Product id = 11032
Application Number = 19826
Date of Application = 14,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE 0.16% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 160MG/100ML
----
Product id = 11034
Application Number = 19826
Date of Application = 14,, Aug, 1992
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.2% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG/100ML
----
Product id = 11038
Application Number = 19826
Date of Application = 14,, Aug, 1992
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE 0.4% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 005
Tecode =
Rld = No
Strength = 400MG/100ML
----
Product id = 11035
Application Number = 19826
Date of Application = 14,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE 0.32% AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 006
Tecode = AP
Rld = Yes
Strength = 320MG/100ML
----
Product id = 16689
Application Number = 40034
Date of Application = 28,, Apr, 1995
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 450MG
----
Product id = 786
Application Number = 40052
Date of Application = 14,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 787
Application Number = 40052
Date of Application = 14,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 125MG
----
Product id = 788
Application Number = 40052
Date of Application = 14,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 200MG
----
Product id = 789
Application Number = 40052
Date of Application = 14,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 004
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16695
Application Number = 40086
Date of Application = 15,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = RHODES PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 16684
Application Number = 40539
Date of Application = 27,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 16682
Application Number = 40543
Date of Application = 27,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 16683
Application Number = 40546
Date of Application = 30,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 450MG
----
Product id = 16681
Application Number = 40548
Date of Application = 30,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16691
Application Number = 40560
Date of Application = 21,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = NOSTRUM
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 600MG
----
Product id = 16690
Application Number = 40595
Date of Application = 21,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = NOSTRUM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 770
Application Number = 81034
Date of Application = 28,, Feb, 1992
RX/OTC/DISCN = RX
Tradename = THEO-24
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTIENT PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 400MG
----
Product id = 16696
Application Number = 81236
Date of Application = 9,, Nov, 1992
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 450MG
----
Product id = 1838
Application Number = 83921
Date of Application = 31,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = ELIXOPHYLLIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 4566
Application Number = 84559
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOLIXIR
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = PANRAY
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 4556
Application Number = 84578
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LANOPHYLLIN
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 28613
Application Number = 84726
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYL-225
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORTHO MCNEIL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 225MG
----
Product id = 3556
Application Number = 84731
Date of Application = 7,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SCHERER RP
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 3555
Application Number = 84731
Date of Application = 7,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SCHERER RP
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 3557
Application Number = 84731
Date of Application = 7,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SCHERER RP
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----
Product id = 4574
Application Number = 84739
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 9139
Application Number = 84875
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MERSALYL-THEOPHYLLINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML;50MG/ML
----
Product id = 309
Application Number = 85075
Date of Application = 24,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = AEROLATE SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = FLEMING PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 260MG
----
Product id = 308
Application Number = 85075
Date of Application = 24,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = AEROLATE JR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = FLEMING PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 130MG
----
Product id = 307
Application Number = 85075
Date of Application = 24,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = AEROLATE III
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = FLEMING PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 65MG
----
Product id = 4570
Application Number = 85169
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 12831
Application Number = 85186
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ELIXOPHYLLIN
Route/format = ORAL / SOLUTION, ELIXIR
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 80MG/15ML
----
Product id = 15147
Application Number = 85187
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SLO-PHYLLIN
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 28083
Application Number = 85202
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SLO-PHYLLIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 750
Application Number = 85203
Date of Application = 24,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = SLO-PHYLLIN
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 28084
Application Number = 85204
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SLO-PHYLLIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 751
Application Number = 85205
Date of Application = 24,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = SLO-PHYLLIN
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 749
Application Number = 85206
Date of Application = 24,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = SLO-PHYLLIN
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 3553
Application Number = 85263
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = KV PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 3554
Application Number = 85263
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = KV PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 1164
Application Number = 85264
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRONKODYL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 1165
Application Number = 85264
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRONKODYL
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 16670
Application Number = 85328
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 16672
Application Number = 85328
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 28610
Application Number = 85353
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOCLEAR-200
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 28609
Application Number = 85353
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOCLEAR-100
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CENT PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 14678
Application Number = 85502
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ELIXICON
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 1837
Application Number = 85545
Date of Application = 31,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = ELIXOPHYLLIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 16652
Application Number = 85665
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SUSTAIRE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ROERIG
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 16653
Application Number = 85665
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SUSTAIRE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ROERIG
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 4573
Application Number = 85863
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = PRECISION DOSE
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 4571
Application Number = 85952
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 775
Application Number = 85983
Date of Application = 20,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOBID
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WHITBY
ProductNo = 001
Tecode =
Rld = No
Strength = 260MG
----
Product id = 15156
Application Number = 86001
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 14050
Application Number = 86107
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOLAIR
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = 3M
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 16678
Application Number = 86363
Date of Application = 16,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = THEOLAIR-SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = 3M
ProductNo = 002
Tecode =
Rld = No
Strength = 250MG
----
Product id = 28611
Application Number = 86399
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = THEOLAIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MEDICIS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 125MG
----
Product id = 28612
Application Number = 86399
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = THEOLAIR
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MEDICIS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 250MG
----
Product id = 780
Application Number = 86471
Date of Application = 8,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOPHYL-SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ORTHO MCNEIL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 779
Application Number = 86480
Date of Application = 8,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOPHYL-SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ORTHO MCNEIL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 4567
Application Number = 86485
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYL-225
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = ORTHO MCNEIL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 112.5MG/15ML
----
Product id = 15369
Application Number = 86506
Date of Application = 12,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOPHYL
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = ORTHO MCNEIL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 15157
Application Number = 86545
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG/15ML
----
Product id = 777
Application Number = 86569
Date of Application = 27,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOCLEAR L.A.-130
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHWARZ PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 130MG
----
Product id = 778
Application Number = 86569
Date of Application = 27,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOCLEAR L.A.-260
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHWARZ PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = 260MG
----
Product id = 4572
Application Number = 86720
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 4576
Application Number = 86748
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 501
Application Number = 86826
Date of Application = 29,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = ELIXOPHYLLIN SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 502
Application Number = 86826
Date of Application = 29,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = ELIXOPHYLLIN SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 250MG
----
Product id = 16671
Application Number = 86998
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 792
Application Number = 87010
Date of Application = 31,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOVENT
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 15155
Application Number = 87095
Date of Application = 1,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOCLEAR-80
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = CENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 3301
Application Number = 87155
Date of Application = 25,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-T
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = FISONS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 3302
Application Number = 87155
Date of Application = 25,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-T
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = FISONS
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 3303
Application Number = 87155
Date of Application = 25,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-T
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = FISONS
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----
Product id = 755
Application Number = 87193
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-CRT
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = GRAHAM DM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 753
Application Number = 87194
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-CRT
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = GRAHAM DM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 16059
Application Number = 87225
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LABID
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 16676
Application Number = 87400
Date of Application = 11,, Jan, 1983
RX/OTC/DISCN = DISCN
Tradename = THEOCHRON
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 14051
Application Number = 87449
Date of Application = 15,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 790
Application Number = 87462
Date of Application = 11,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 260MG
----
Product id = 16554
Application Number = 87563
Date of Application = 21,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = QUIBRON-T/SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MONARCH PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16741
Application Number = 87571
Date of Application = 1,, Sep, 1982
RX/OTC/DISCN = RX
Tradename = UNIPHYL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = RHODES PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 4569
Application Number = 87679
Date of Application = 15,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = CENCI
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 752
Application Number = 87763
Date of Application = 27,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-CRT
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = GRAHAM DM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 776
Application Number = 87854
Date of Application = 20,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOBID JR.
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WHITBY
ProductNo = 001
Tecode =
Rld = No
Strength = 130MG
----
Product id = 745
Application Number = 87892
Date of Application = 31,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = SLO-BID
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 747
Application Number = 87893
Date of Application = 31,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = SLO-BID
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 748
Application Number = 87894
Date of Application = 31,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = SLO-BID
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 793
Application Number = 87910
Date of Application = 31,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOVENT
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 14901
Application Number = 87917
Date of Application = 18,, Jan, 1983
RX/OTC/DISCN = DISCN
Tradename = AQUAPHYLLIN
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = FERNDALE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 767
Application Number = 87942
Date of Application = 22,, Aug, 1983
RX/OTC/DISCN = RX
Tradename = THEO-24
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTIENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 768
Application Number = 87943
Date of Application = 22,, Aug, 1983
RX/OTC/DISCN = RX
Tradename = THEO-24
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTIENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 769
Application Number = 87944
Date of Application = 22,, Aug, 1983
RX/OTC/DISCN = RX
Tradename = THEO-24
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTIENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 774
Application Number = 87995
Date of Application = 10,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 772
Application Number = 88015
Date of Application = 10,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 75MG
----
Product id = 773
Application Number = 88016
Date of Application = 10,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 771
Application Number = 88022
Date of Application = 10,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 16654
Application Number = 88253
Date of Application = 17,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = T-PHYL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PHARM RES ASSOC
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 791
Application Number = 88255
Date of Application = 12,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE-SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERER RP
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 743
Application Number = 88269
Date of Application = 31,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = SLO-BID
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 4551
Application Number = 88303
Date of Application = 25,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = ELIXOMIN
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = CENCI
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 16674
Application Number = 88320
Date of Application = 21,, Feb, 1985
RX/OTC/DISCN = RX
Tradename = THEOCHRON
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 16675
Application Number = 88321
Date of Application = 21,, Feb, 1985
RX/OTC/DISCN = RX
Tradename = THEOCHRON
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 16679
Application Number = 88364
Date of Application = 16,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = THEOLAIR-SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = 3M
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16677
Application Number = 88369
Date of Application = 16,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = THEOLAIR-SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = 3M
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 754
Application Number = 88382
Date of Application = 27,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-CRT
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = GRAHAM DM
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 756
Application Number = 88383
Date of Application = 27,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = SOMOPHYLLIN-CRT
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = GRAHAM DM
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 15897
Application Number = 88503
Date of Application = 3,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = DURAPHYL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 15898
Application Number = 88504
Date of Application = 3,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = DURAPHYL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 15899
Application Number = 88505
Date of Application = 3,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = DURAPHYL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = FOREST LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 781
Application Number = 88654
Date of Application = 12,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = CENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 27172
Application Number = 88656
Date of Application = 22,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = QUIBRON-T
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MONARCH PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 782
Application Number = 88689
Date of Application = 12,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = CENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 14873
Application Number = 88746
Date of Application = 22,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = ACCURBRON
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG/15ML
----
Product id = 16673
Application Number = 89131
Date of Application = 25,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = THEO-DUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 450MG
----
Product id = 16680
Application Number = 89132
Date of Application = 16,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = THEOLAIR-SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = 3M
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 13733
Application Number = 89141
Date of Application = 3,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = AEROLATE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = FLEMING PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG/15ML
----
Product id = 4568
Application Number = 89223
Date of Application = 27,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 744
Application Number = 89539
Date of Application = 10,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = SLO-BID
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 75MG
----
Product id = 746
Application Number = 89540
Date of Application = 10,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = SLO-BID
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 4575
Application Number = 89626
Date of Application = 28,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / ELIXIR
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 80MG/15ML
----
Product id = 16694
Application Number = 89763
Date of Application = 30,, Apr, 1990
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 16692
Application Number = 89807
Date of Application = 30,, Apr, 1990
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 16693
Application Number = 89808
Date of Application = 30,, Apr, 1990
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 16739
Application Number = 89822
Date of Application = 4,, Jan, 1995
RX/OTC/DISCN = DISCN
Tradename = UNI-DUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 16740
Application Number = 89823
Date of Application = 4,, Jan, 1995
RX/OTC/DISCN = DISCN
Tradename = UNI-DUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 785
Application Number = 89932
Date of Application = 4,, Jan, 1995
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 783
Application Number = 89976
Date of Application = 4,, Jan, 1995
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 784
Application Number = 89977
Date of Application = 4,, Jan, 1995
RX/OTC/DISCN = DISCN
Tradename = THEOPHYLLINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 16687
Application Number = 90355
Date of Application = 13,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 16688
Application Number = 90355
Date of Application = 13,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 16685
Application Number = 90430
Date of Application = 27,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 16686
Application Number = 90430
Date of Application = 27,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 450MG
----
Product id = 14052
Application Number = 91156
Date of Application = 13,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = SILARX
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 80MG/15ML
----
Product id = 14053
Application Number = 91586
Date of Application = 15,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = THEOPHYLLINE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 80MG/15ML
----