terbutaline-sulfate
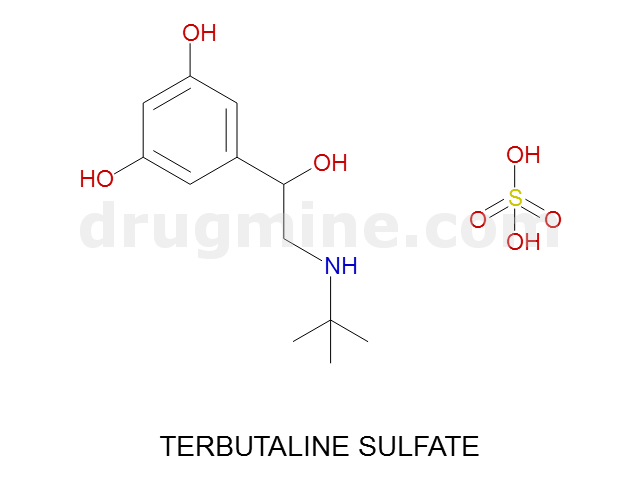


Name: TERBUTALINE SULFATE
ID :
MW: 225
Number of atoms: 16
Molecular_Formula: C12H19NO3
Alogp: 1.304
Indication class : Bronchodilator
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
terbutaline sulfate containing products summary
There are in total 18 different products containing the active ingredient terbutaline sulfate. From the 18 drug products, 9 have been discontinued.Product id = 6171
Application Number = 17466
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRICANYL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 18615
Application Number = 17618
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRICANYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 18616
Application Number = 17618
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRICANYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18613
Application Number = 17849
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRETHINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LEHIGH VALLEY
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 18614
Application Number = 17849
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRETHINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LEHIGH VALLEY
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 39
Application Number = 18000
Date of Application = 19,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = BRICANYL
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 0.2MG/INH
----
Product id = 6138
Application Number = 18571
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = BRETHINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = AAIPHARMA LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 38
Application Number = 18762
Date of Application = 17,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = BRETHAIRE
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.2MG/INH
----
Product id = 28592
Application Number = 75877
Date of Application = 26,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 28593
Application Number = 75877
Date of Application = 26,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 10964
Application Number = 76770
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 1MG/ML
----
Product id = 10967
Application Number = 76853
Date of Application = 20,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = TERBUTALINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 10965
Application Number = 76887
Date of Application = 26,, May, 2004
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 28594
Application Number = 77152
Date of Application = 25,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 28595
Application Number = 77152
Date of Application = 25,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 10963
Application Number = 78151
Date of Application = 7,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 10966
Application Number = 78630
Date of Application = 20,, May, 2009
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 10968
Application Number = 200122
Date of Application = 8,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = TERBUTALINE SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = UNITED BIOMEDCL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----