temazepam
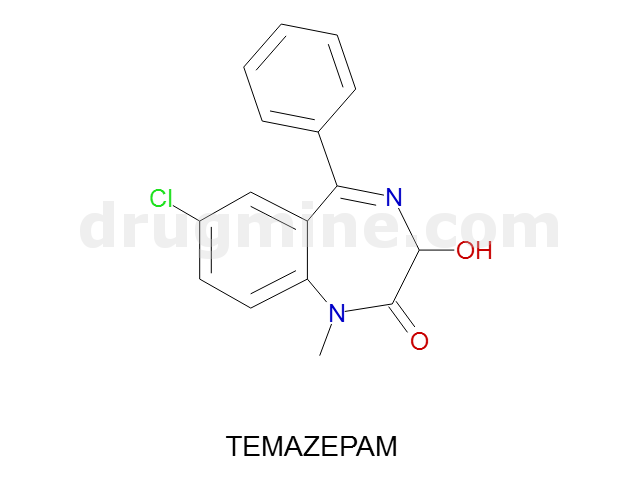
Name: TEMAZEPAM
ID :
MW: 301
Number of atoms: 21
Molecular_Formula: C16H13ClN2O2
Alogp: 3.05
Indication class : Tranquilizer (minor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
temazepam containing products summary
There are in total 30 different products containing the active ingredient temazepam. From the 30 drug products, 12 have been discontinued.Product id = 3164
Application Number = 18163
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RESTORIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 3166
Application Number = 18163
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RESTORIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 30MG
----
Product id = 3163
Application Number = 18163
Date of Application = 25,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = RESTORIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 7.5MG
----
Product id = 3165
Application Number = 18163
Date of Application = 2,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = RESTORIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 22.5MG
----
Product id = 3437
Application Number = 70383
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG
----
Product id = 3439
Application Number = 70384
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 3435
Application Number = 70489
Date of Application = 7,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG
----
Product id = 3436
Application Number = 70490
Date of Application = 7,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 3416
Application Number = 70547
Date of Application = 15,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = TEMAZ
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 3415
Application Number = 70564
Date of Application = 15,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = TEMAZ
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG
----
Product id = 3428
Application Number = 70920
Date of Application = 10,, Jul, 1986
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 3425
Application Number = 70920
Date of Application = 21,, May, 2010
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 7.5MG
----
Product id = 3427
Application Number = 70920
Date of Application = 12,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 22.5MG
----
Product id = 3426
Application Number = 70920
Date of Application = 7,, Jul, 1986
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 3422
Application Number = 71174
Date of Application = 10,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG
----
Product id = 3424
Application Number = 71175
Date of Application = 10,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 3423
Application Number = 71175
Date of Application = 14,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 22.5MG
----
Product id = 3433
Application Number = 71427
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 3434
Application Number = 71428
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 3438
Application Number = 71446
Date of Application = 21,, May, 1993
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG
----
Product id = 3440
Application Number = 71447
Date of Application = 21,, May, 1993
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 3430
Application Number = 71456
Date of Application = 21,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 3432
Application Number = 71457
Date of Application = 21,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 3429
Application Number = 71457
Date of Application = 22,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 7.5MG
----
Product id = 3431
Application Number = 71457
Date of Application = 22,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 22.5MG
----
Product id = 3418
Application Number = 71620
Date of Application = 7,, Aug, 1987
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 3417
Application Number = 71638
Date of Application = 7,, Aug, 1987
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 3419
Application Number = 71708
Date of Application = 29,, Sep, 1988
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG
----
Product id = 3420
Application Number = 71709
Date of Application = 29,, Sep, 1988
RX/OTC/DISCN = DISCN
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 3421
Application Number = 78581
Date of Application = 8,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = TEMAZEPAM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 7.5MG
----