sulindac
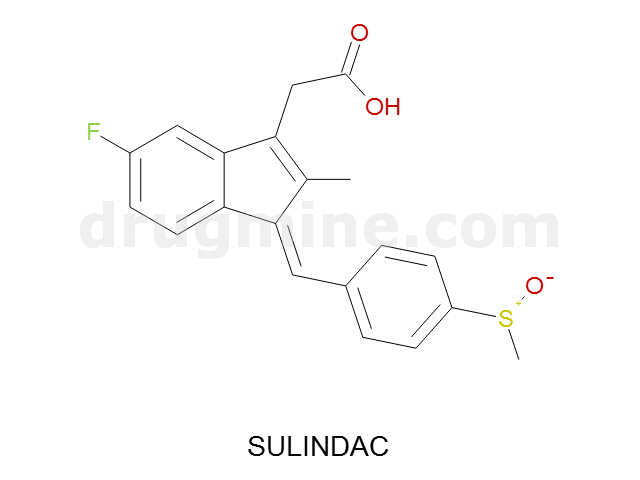
Name: SULINDAC
ID :
MW: 356
Number of atoms: 25
Molecular_Formula: C20H17FO3S
Alogp: 3.956
Indication class : Anti-Inflammatory
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
sulindac containing products summary
There are in total 16 different products containing the active ingredient sulindac. From the 16 drug products, 5 have been discontinued.Product id = 19761
Application Number = 17911
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLINORIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 19762
Application Number = 17911
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CLINORIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 28370
Application Number = 71795
Date of Application = 3,, Apr, 1990
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 28369
Application Number = 71891
Date of Application = 3,, Apr, 1990
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 28361
Application Number = 72050
Date of Application = 17,, Apr, 1991
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 28362
Application Number = 72051
Date of Application = 17,, Apr, 1991
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 28357
Application Number = 72710
Date of Application = 25,, Mar, 1991
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EPIC PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 28358
Application Number = 72711
Date of Application = 25,, Mar, 1991
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EPIC PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 28365
Application Number = 72712
Date of Application = 30,, Aug, 1991
RX/OTC/DISCN = DISCN
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 28366
Application Number = 72713
Date of Application = 30,, Aug, 1991
RX/OTC/DISCN = DISCN
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 28367
Application Number = 72972
Date of Application = 28,, Feb, 1992
RX/OTC/DISCN = DISCN
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 28368
Application Number = 72973
Date of Application = 28,, Feb, 1992
RX/OTC/DISCN = DISCN
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 28364
Application Number = 73039
Date of Application = 22,, Jun, 1993
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 28363
Application Number = 73039
Date of Application = 22,, Jun, 1993
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 28360
Application Number = 73262
Date of Application = 6,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 28359
Application Number = 73262
Date of Application = 6,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = SULINDAC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----