sulfasalazine
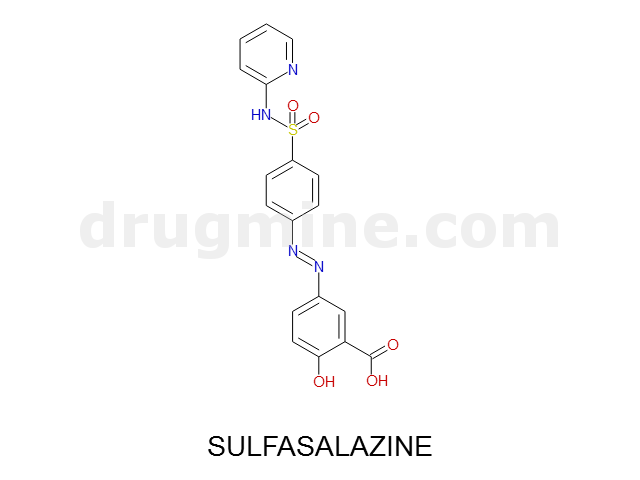
Name: SULFASALAZINE
ID :
MW: 398
Number of atoms: 28
Molecular_Formula: C18H14N4O5S
Alogp: 3.463
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
sulfasalazine containing products summary
There are in total 15 different products containing the active ingredient sulfasalazine. From the 15 drug products, 9 have been discontinued.Product id = 18282
Application Number = 7073
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = AZULFIDINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 15400
Application Number = 7073
Date of Application = 6,, Apr, 1983
RX/OTC/DISCN = RX
Tradename = AZULFIDINE EN-TABS
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 14642
Application Number = 18605
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = AZULFIDINE
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG/5ML
----
Product id = 28332
Application Number = 40349
Date of Application = 11,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 15607
Application Number = 75339
Date of Application = 11,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 28328
Application Number = 80197
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 27816
Application Number = 83450
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = S.A.S.-500
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 28333
Application Number = 84964
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 28334
Application Number = 85828
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 28330
Application Number = 86184
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 14641
Application Number = 86983
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = AZULFIDINE
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 250MG/5ML
----
Product id = 28335
Application Number = 87197
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 15608
Application Number = 88052
Date of Application = 24,, May, 1983
RX/OTC/DISCN = DISCN
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 28331
Application Number = 89339
Date of Application = 26,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 28329
Application Number = 89590
Date of Application = 19,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = SULFASALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----