sodium-lactate
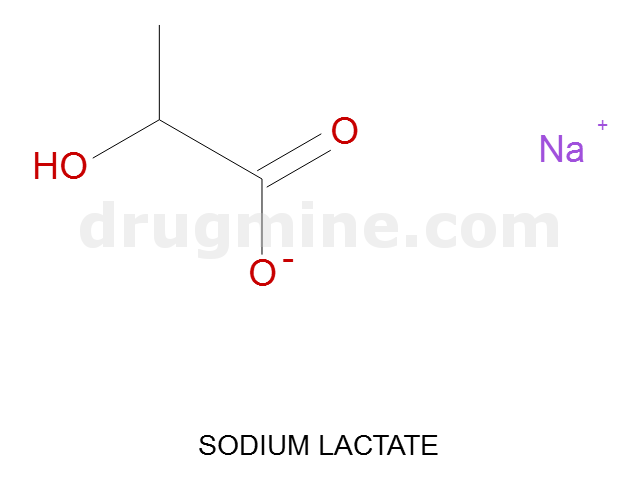
Name: SODIUM LACTATE
ID :
MW: 89
Number of atoms: 6
Molecular_Formula: C3H5O3
Alogp: -1.867
Indication class : Replenisher (electrolyte)
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
sodium lactate containing products summary
There are in total 93 different products containing the active ingredient sodium lactate. From the 93 drug products, 23 have been discontinued.Product id = 8682
Application Number = 16679
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 8686
Application Number = 16682
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10834
Application Number = 16692
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SODIUM LACTATE 0.167 MOLAR IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1.87GM/100ML
----
Product id = 10172
Application Number = 17390
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PLASMA-LYTE M AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 37MG/100ML;5GM/100ML;30MG/100ML;119MG/100ML;161MG/100ML;94MG/100ML;138MG/100ML
----
Product id = 10173
Application Number = 17438
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PLASMA-LYTE R IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 36.8MG/100ML;30.5MG/100ML;74.6MG/100ML;640MG/100ML;496MG/100ML;89.6MG/100ML
----
Product id = 7092
Application Number = 17484
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DEXTROSE 5% AND ELECTROLYTE NO.48 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 5GM/100ML;31MG/100ML;141MG/100ML;20MG/100ML;12MG/100ML;260MG/100ML
----
Product id = 7122
Application Number = 17510
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DEXTROSE 5% IN LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 13501
Application Number = 17512
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DIANEAL 137 W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13503
Application Number = 17512
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DIANEAL 137 W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13502
Application Number = 17512
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DIANEAL 137 W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13512
Application Number = 17512
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-2 W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode = AT
Rld = No
Strength = 18.3MG/100ML;1.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13514
Application Number = 17512
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-2 W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 005
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13517
Application Number = 17512
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-2 W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 006
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13508
Application Number = 17512
Date of Application = 9,, Jul, 1984
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-1 W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 007
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13509
Application Number = 17512
Date of Application = 9,, Jul, 1984
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-1 W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 008
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13511
Application Number = 17512
Date of Application = 9,, Jul, 1984
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-1 W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 009
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13510
Application Number = 17512
Date of Application = 18,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-1 W/ DEXTROSE 3.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 010
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;3.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13516
Application Number = 17512
Date of Application = 18,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-2 W/ DEXTROSE 3.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 011
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;3.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 7093
Application Number = 17608
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 8687
Application Number = 17641
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 8684
Application Number = 18023
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10833
Application Number = 18186
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SODIUM LACTATE 0.167 MOLAR IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 1.87GM/100ML
----
Product id = 10835
Application Number = 18249
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SODIUM LACTATE 0.167 MOLAR IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 1.87GM/100ML
----
Product id = 13480
Application Number = 18379
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 001
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13471
Application Number = 18379
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 002
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13475
Application Number = 18379
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 003
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13484
Application Number = 18379
Date of Application = 7,, Jul, 1982
RX/OTC/DISCN = RX
Tradename = DELFLEX-LM W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 004
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13485
Application Number = 18379
Date of Application = 7,, Jul, 1982
RX/OTC/DISCN = RX
Tradename = DELFLEX-LM W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 005
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13487
Application Number = 18379
Date of Application = 7,, Jul, 1982
RX/OTC/DISCN = RX
Tradename = DELFLEX-LM W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 006
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13479
Application Number = 18379
Date of Application = 24,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 3.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 007
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;3.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13486
Application Number = 18379
Date of Application = 24,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = DELFLEX-LM W/ DEXTROSE 3.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 008
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;3.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 8688
Application Number = 18417
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MILES
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 13493
Application Number = 18460
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIALYTE LM/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = No
Strength = 26MG/100ML;1.5GM/100ML;15MG/100ML;560MG/100ML;390MG/100ML
----
Product id = 13498
Application Number = 18460
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIALYTE LM/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 004
Tecode =
Rld = No
Strength = 26MG/100ML;4.25GM/100ML;15MG/100ML;560MG/100ML;390MG/100ML
----
Product id = 13494
Application Number = 18460
Date of Application = 2,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = DIALYTE LM/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 005
Tecode =
Rld = No
Strength = 26MG/100ML;2.5GM/100ML;5MG/100ML;530MG/100ML;450MG/100ML
----
Product id = 13492
Application Number = 18460
Date of Application = 29,, Jan, 1986
RX/OTC/DISCN = RX
Tradename = DIALYTE LM/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 007
Tecode =
Rld = No
Strength = 26MG/100ML;1.5GM/100ML;5MG/100ML;530MG/100ML;450MG/100ML
----
Product id = 13495
Application Number = 18460
Date of Application = 29,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = DIALYTE LM/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 008
Tecode =
Rld = No
Strength = 26MG/100ML;5GM/100ML;5MG/100ML;530MG/100ML;450MG/100ML
----
Product id = 13497
Application Number = 18460
Date of Application = 29,, Jan, 1986
RX/OTC/DISCN = RX
Tradename = DIALYTE LM/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 009
Tecode =
Rld = No
Strength = 26MG/100ML;4.25GM/100ML;5MG/100ML;530MG/100ML;450MG/100ML
----
Product id = 13636
Application Number = 18494
Date of Application = 19,, Feb, 1982
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AT
Rld = No
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 7124
Application Number = 18499
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DEXTROSE 5% IN LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MILES
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 13635
Application Number = 18681
Date of Application = 27,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 7091
Application Number = 18840
Date of Application = 29,, Jun, 1983
RX/OTC/DISCN = RX
Tradename = DEXTROSE 5% AND ELECTROLYTE NO 75 IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 5GM/100ML;205MG/100ML;100MG/100ML;120MG/100ML;220MG/100ML
----
Product id = 13472
Application Number = 18883
Date of Application = 30,, Nov, 1984
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 001
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13476
Application Number = 18883
Date of Application = 30,, Nov, 1984
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 002
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13481
Application Number = 18883
Date of Application = 30,, Nov, 1984
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 003
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;15.2MG/100ML;567MG/100ML;392MG/100ML
----
Product id = 13473
Application Number = 18883
Date of Application = 30,, Nov, 1984
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 1.5% LOW MAGNESIUM IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 004
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13477
Application Number = 18883
Date of Application = 30,, Nov, 1984
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 2.5% LOW MAGNESIUM IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 005
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13482
Application Number = 18883
Date of Application = 30,, Nov, 1984
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 4.25% LOW MAGNESIUM IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 006
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13637
Application Number = 18921
Date of Application = 3,, Apr, 1984
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10837
Application Number = 18947
Date of Application = 5,, Sep, 1984
RX/OTC/DISCN = RX
Tradename = SODIUM LACTATE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MEQ/ML
----
Product id = 10381
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 5MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;105MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10311
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 10MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;105MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10312
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 10MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;179MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10337
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 20MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;179MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10338
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 20MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 005
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;328MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10331
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 15MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 006
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;254MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10358
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 30MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 007
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;254MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10369
Application Number = 19367
Date of Application = 5,, Apr, 1985
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 40MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 008
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;328MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 13524
Application Number = 19395
Date of Application = 26,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = INPERSOL-ZM W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 001
Tecode =
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;538MG/100ML;448MG/100ML
----
Product id = 13525
Application Number = 19395
Date of Application = 26,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = INPERSOL-ZM W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 002
Tecode =
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;538MG/100ML;448MG/100ML
----
Product id = 13526
Application Number = 19395
Date of Application = 26,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = INPERSOL-ZM W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 003
Tecode =
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;538MG/100ML;448MG/100ML
----
Product id = 13639
Application Number = 19416
Date of Application = 17,, Jan, 1986
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 8683
Application Number = 19485
Date of Application = 24,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 8431
Application Number = 19513
Date of Application = 8,, May, 1986
RX/OTC/DISCN = RX
Tradename = IONOSOL MB AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 5GM/100ML;30MG/100ML;141MG/100ML;15MG/100ML;260MG/100ML;25MG/100ML
----
Product id = 8430
Application Number = 19515
Date of Application = 8,, May, 1986
RX/OTC/DISCN = RX
Tradename = IONOSOL B AND DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 5GM/100ML;53MG/100ML;100MG/100ML;100MG/100ML;180MG/100ML;280MG/100ML;16MG/100ML
----
Product id = 8685
Application Number = 19632
Date of Application = 29,, Feb, 1988
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 7077
Application Number = 19634
Date of Application = 24,, Feb, 1988
RX/OTC/DISCN = RX
Tradename = DEXTROSE 2.5% IN HALF-STRENGTH LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/100ML;2.5GM/100ML;15MG/100ML;300MG/100ML;160MG/100ML
----
Product id = 7088
Application Number = 19634
Date of Application = 24,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = DEXTROSE 4% IN MODIFIED LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/100ML;4GM/100ML;6MG/100ML;120MG/100ML;62MG/100ML
----
Product id = 7123
Application Number = 19634
Date of Application = 24,, Feb, 1988
RX/OTC/DISCN = RX
Tradename = DEXTROSE 5% IN LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 003
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10382
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = POTASSIUM CHLORIDE 5MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;104MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10339
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 20MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;179MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10359
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = POTASSIUM CHLORIDE 30MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;254MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10370
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 40MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 004
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;328MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10313
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = POTASSIUM CHLORIDE 10MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 005
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;104MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10314
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = POTASSIUM CHLORIDE 10MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 006
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;179MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10332
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = POTASSIUM CHLORIDE 15MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 007
Tecode =
Rld = No
Strength = 20MG/100ML;5GM/100ML;254MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10340
Application Number = 19685
Date of Application = 17,, Oct, 1988
RX/OTC/DISCN = RX
Tradename = POTASSIUM CHLORIDE 20MEQ IN DEXTROSE 5% AND LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 008
Tecode = AP
Rld = No
Strength = 20MG/100ML;5GM/100ML;328MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 13638
Application Number = 19933
Date of Application = 29,, Aug, 1989
RX/OTC/DISCN = RX
Tradename = LACTATED RINGER'S IN PLASTIC CONTAINER
Route/format = IRRIGATION / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 20MG/100ML;30MG/100ML;600MG/100ML;310MG/100ML
----
Product id = 10836
Application Number = 20004
Date of Application = 21,, Apr, 1992
RX/OTC/DISCN = RX
Tradename = SODIUM LACTATE 1/6 MOLAR IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1.87GM/100ML
----
Product id = 13513
Application Number = 20163
Date of Application = 4,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-2 W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;1.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13515
Application Number = 20163
Date of Application = 4,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-2 W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;2.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13518
Application Number = 20163
Date of Application = 4,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DIANEAL PD-2 W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode = AT
Rld = No
Strength = 25.7MG/100ML;4.25GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13474
Application Number = 20171
Date of Application = 19,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 1.5% LOW MAGNESIUM LOW CALCIUM IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 001
Tecode = AT
Rld = No
Strength = 18.4MG/100ML;1.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13478
Application Number = 20171
Date of Application = 19,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 2.5% LOW MAGNESIUM LOW CALCIUM IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 002
Tecode = AT
Rld = No
Strength = 18.4MG/100ML;2.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13483
Application Number = 20171
Date of Application = 19,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = DELFLEX W/ DEXTROSE 4.25% LOW MAGNESIUM LOW CALCIUM IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = FRESENIUS MEDCL
ProductNo = 003
Tecode = AT
Rld = No
Strength = 18.4MG/100ML;4.25GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13504
Application Number = 20183
Date of Application = 4,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DIANEAL LOW CALCIUM W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AT
Rld = No
Strength = 18.3MG/100ML;1.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13505
Application Number = 20183
Date of Application = 4,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DIANEAL LOW CALCIUM W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 18.3MG/100ML;2.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13506
Application Number = 20183
Date of Application = 4,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DIANEAL LOW CALCIUM W/ DEXTROSE 3.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = 18.3MG/100ML;3.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13507
Application Number = 20183
Date of Application = 4,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DIANEAL LOW CALCIUM W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode =
Rld = No
Strength = 18.3MG/100ML;4.25GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13520
Application Number = 20374
Date of Application = 13,, Jun, 1994
RX/OTC/DISCN = DISCN
Tradename = INPERSOL-LC/LM W/ DEXTROSE 1.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = A
Applicant Name = FRESENIUS
ProductNo = 001
Tecode =
Rld = No
Strength = 18.4MG/100ML;1.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13521
Application Number = 20374
Date of Application = 13,, Jun, 1994
RX/OTC/DISCN = DISCN
Tradename = INPERSOL-LC/LM W/ DEXTROSE 2.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = A
Applicant Name = FRESENIUS
ProductNo = 002
Tecode =
Rld = No
Strength = 18.4MG/100ML;2.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13522
Application Number = 20374
Date of Application = 13,, Jun, 1994
RX/OTC/DISCN = DISCN
Tradename = INPERSOL-LC/LM W/ DEXTROSE 3.5% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = A
Applicant Name = FRESENIUS
ProductNo = 003
Tecode =
Rld = No
Strength = 18.4MG/100ML;3.5GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----
Product id = 13523
Application Number = 20374
Date of Application = 13,, Jun, 1994
RX/OTC/DISCN = DISCN
Tradename = INPERSOL-LC/LM W/ DEXTROSE 4.25% IN PLASTIC CONTAINER
Route/format = INTRAPERITONEAL / SOLUTION
Application Type = A
Applicant Name = FRESENIUS
ProductNo = 004
Tecode =
Rld = No
Strength = 18.4MG/100ML;4.25GM/100ML;5.08MG/100ML;538MG/100ML;448MG/100ML
----