rotigotine
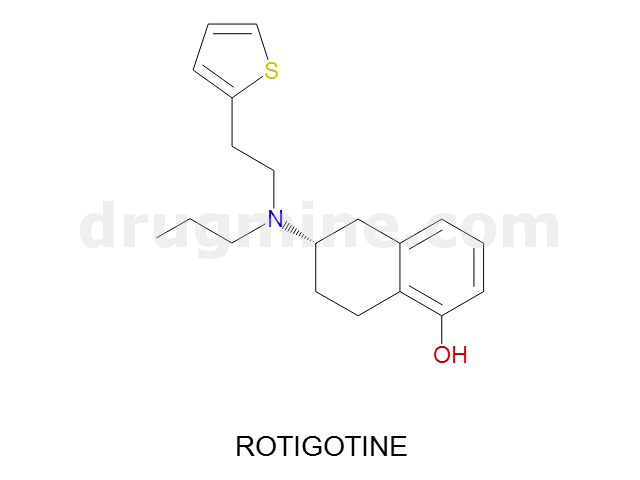
Name: ROTIGOTINE
ID :
MW: 315
Number of atoms: 22
Molecular_Formula: C19H25NOS
Alogp: 4.986
Indication class : .
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
rotigotine containing products summary
There are in total 6 different products containing the active ingredient rotigotine. From the 6 drug products, zero have been discontinued.Product id = 4731
Application Number = 21829
Date of Application = 9,, May, 2007
RX/OTC/DISCN = RX
Tradename = NEUPRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2MG/24HR
----
Product id = 4733
Application Number = 21829
Date of Application = 9,, May, 2007
RX/OTC/DISCN = RX
Tradename = NEUPRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG/24HR
----
Product id = 4734
Application Number = 21829
Date of Application = 9,, May, 2007
RX/OTC/DISCN = RX
Tradename = NEUPRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 003
Tecode =
Rld = No
Strength = 6MG/24HR
----
Product id = 4730
Application Number = 21829
Date of Application = 2,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = NEUPRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 004
Tecode =
Rld = No
Strength = 1MG/24HR
----
Product id = 4732
Application Number = 21829
Date of Application = 2,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = NEUPRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 005
Tecode =
Rld = No
Strength = 3MG/24HR
----
Product id = 4735
Application Number = 21829
Date of Application = 2,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = NEUPRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 006
Tecode =
Rld = No
Strength = 8MG/24HR
----