rosiglitazone-maleate
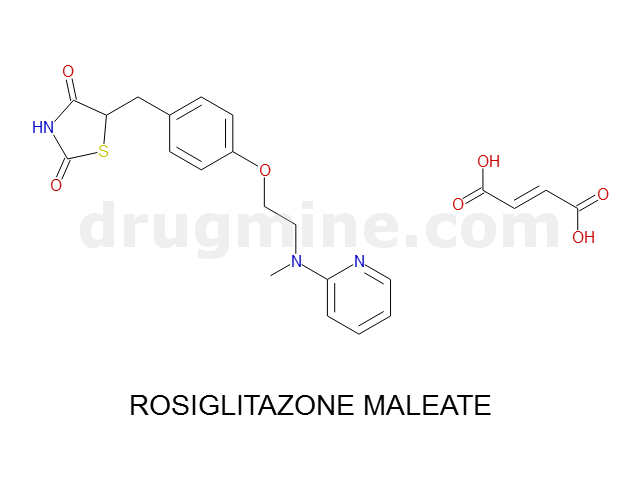
Name: ROSIGLITAZONE MALEATE
ID :
MW: 357
Number of atoms: 25
Molecular_Formula: C18H19N3O3S
Alogp: 3.268
Indication class : Antidiabetic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
rosiglitazone maleate containing products summary
There are in total 20 different products containing the active ingredient rosiglitazone maleate. From the 20 drug products, 1 have been discontinued.Product id = 18232
Application Number = 21071
Date of Application = 25,, May, 1999
RX/OTC/DISCN = RX
Tradename = AVANDIA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 18233
Application Number = 21071
Date of Application = 25,, May, 1999
RX/OTC/DISCN = RX
Tradename = AVANDIA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 18234
Application Number = 21071
Date of Application = 25,, May, 1999
RX/OTC/DISCN = RX
Tradename = AVANDIA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 8MG BASE
----
Product id = 18224
Application Number = 21410
Date of Application = 10,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;EQ 1MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18225
Application Number = 21410
Date of Application = 10,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG;EQ 2MG BASE
----
Product id = 18226
Application Number = 21410
Date of Application = 10,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG;EQ 4MG BASE
----
Product id = 18222
Application Number = 21410
Date of Application = 25,, Aug, 2003
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 004
Tecode = AB
Rld = No
Strength = 1GM;EQ 2MG BASE
----
Product id = 18223
Application Number = 21410
Date of Application = 25,, Aug, 2003
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 1GM;EQ 4MG BASE
----
Product id = 18227
Application Number = 21700
Date of Application = 23,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1MG;4MG
----
Product id = 18228
Application Number = 21700
Date of Application = 23,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG;4MG
----
Product id = 18230
Application Number = 21700
Date of Application = 23,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 003
Tecode =
Rld = No
Strength = 4MG;4MG
----
Product id = 18229
Application Number = 21700
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG;8MG
----
Product id = 18231
Application Number = 21700
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = AVANDARYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 005
Tecode =
Rld = No
Strength = 4MG;8MG
----
Product id = 27798
Application Number = 76747
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27799
Application Number = 76747
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 27800
Application Number = 76747
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 27803
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 2MG BASE
----
Product id = 27804
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG;EQ 4MG BASE
----
Product id = 27802
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM;EQ 2MG BASE
----
Product id = 27801
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 1GM;EQ 4MG BASE
----