rizatriptan-benzoate
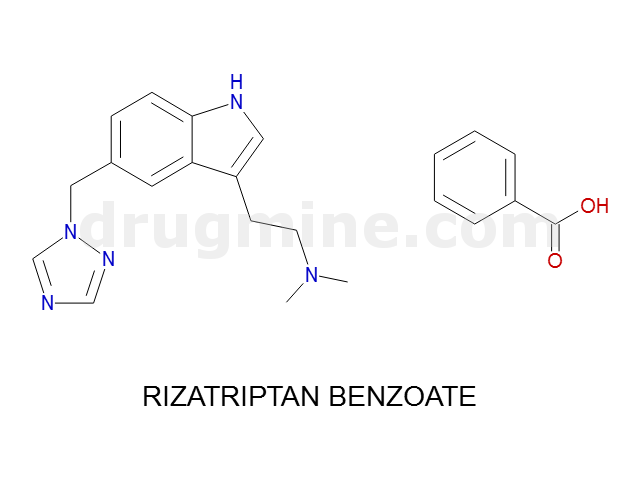
Name: RIZATRIPTAN BENZOATE
ID :
MW: 269
Number of atoms: 20
Molecular_Formula: C15H19N5
Alogp: 2.067
Indication class : Antimigraine
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
rizatriptan benzoate containing products summary
There are in total 44 different products containing the active ingredient rizatriptan benzoate. From the 44 drug products, zero have been discontinued.Product id = 24126
Application Number = 20864
Date of Application = 29,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = MAXALT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24127
Application Number = 20864
Date of Application = 29,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = MAXALT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE
----
Product id = 16927
Application Number = 20865
Date of Application = 29,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = MAXALT-MLT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 16928
Application Number = 20865
Date of Application = 29,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = MAXALT-MLT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE
----
Product id = 27714
Application Number = 77263
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27715
Application Number = 77263
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27694
Application Number = 77526
Date of Application = 26,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27695
Application Number = 77526
Date of Application = 26,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17093
Application Number = 78173
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17094
Application Number = 78173
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17097
Application Number = 78739
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17098
Application Number = 78739
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27710
Application Number = 79230
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27711
Application Number = 79230
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27708
Application Number = 200482
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27709
Application Number = 200482
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17089
Application Number = 201914
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17090
Application Number = 201914
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27698
Application Number = 201967
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27699
Application Number = 201967
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27706
Application Number = 201993
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27707
Application Number = 201993
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27712
Application Number = 202047
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27713
Application Number = 202047
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27690
Application Number = 202244
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27691
Application Number = 202244
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17085
Application Number = 202477
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17086
Application Number = 202477
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27692
Application Number = 202490
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27693
Application Number = 202490
Date of Application = 31,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17087
Application Number = 203062
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17088
Application Number = 203062
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17091
Application Number = 203146
Date of Application = 19,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17092
Application Number = 203146
Date of Application = 19,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27704
Application Number = 203147
Date of Application = 11,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27705
Application Number = 203147
Date of Application = 11,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27702
Application Number = 203252
Date of Application = 31,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27703
Application Number = 203252
Date of Application = 31,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17095
Application Number = 203478
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17096
Application Number = 203478
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27696
Application Number = 204090
Date of Application = 26,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27697
Application Number = 204090
Date of Application = 26,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27700
Application Number = 204339
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27701
Application Number = 204339
Date of Application = 1,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = RIZATRIPTAN BENZOATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----