ribavirin
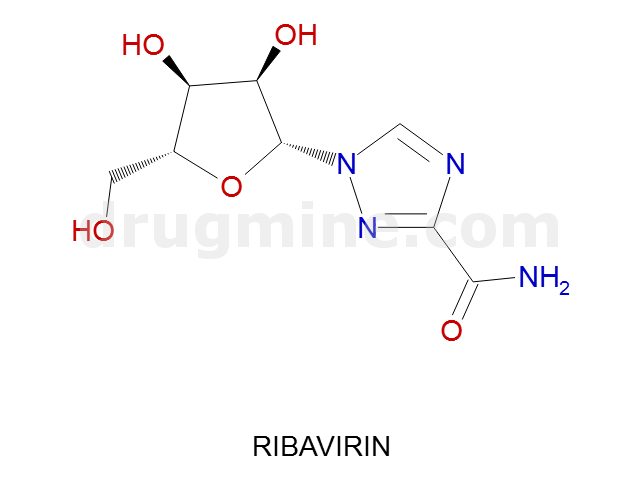
Name: RIBAVIRIN
ID :
MW: 244
Number of atoms: 17
Molecular_Formula: C8H12N4O5
Alogp: -2.745
Indication class : Antiviral
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ribavirin containing products summary
There are in total 25 different products containing the active ingredient ribavirin. From the 25 drug products, 1 have been discontinued.Product id = 4812
Application Number = 18859
Date of Application = 31,, Dec, 1985
RX/OTC/DISCN = RX
Tradename = VIRAZOLE
Route/format = INHALATION / FOR SOLUTION
Application Type = N
Applicant Name = VALEANT PHARM INTL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 6GM/VIAL
----
Product id = 3160
Application Number = 20903
Date of Application = 3,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = REBETOL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode =
Rld = Yes
Strength = 200MG **Indicated for use and comarketed with Interferon ALFA-2B, Recombinant (INTRON A), as Rebetron Combination Therapy**
----
Product id = 3161
Application Number = 20903
Date of Application = 25,, Jul, 2001
RX/OTC/DISCN = RX
Tradename = REBETOL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 19977
Application Number = 21511
Date of Application = 3,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = COPEGUS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 19978
Application Number = 21511
Date of Application = 21,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = COPEGUS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = 400MG
----
Product id = 14017
Application Number = 21546
Date of Application = 29,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = REBETOL
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = Yes
Strength = 40MG/ML
----
Product id = 3182
Application Number = 76192
Date of Application = 6,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 3180
Application Number = 76203
Date of Application = 6,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = RIBASPHERE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = THREE RIVERS PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 3183
Application Number = 76277
Date of Application = 4,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27523
Application Number = 77053
Date of Application = 5,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27527
Application Number = 77094
Date of Application = 5,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27528
Application Number = 77094
Date of Application = 16,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 27530
Application Number = 77094
Date of Application = 16,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 27529
Application Number = 77094
Date of Application = 18,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 3184
Application Number = 77224
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27524
Application Number = 77456
Date of Application = 5,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = THREE RIVERS PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27525
Application Number = 77456
Date of Application = 5,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = THREE RIVERS PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 27526
Application Number = 77456
Date of Application = 5,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = THREE RIVERS PHARMS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 600MG
----
Product id = 27518
Application Number = 77743
Date of Application = 3,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27517
Application Number = 79111
Date of Application = 17,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 3181
Application Number = 79117
Date of Application = 17,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = RIBAVARIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27519
Application Number = 202546
Date of Application = 12,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 27520
Application Number = 202546
Date of Application = 12,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 27521
Application Number = 202546
Date of Application = 12,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 27522
Application Number = 202546
Date of Application = 12,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = RIBAVIRIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 600MG
----