raloxifene-hydrochloride
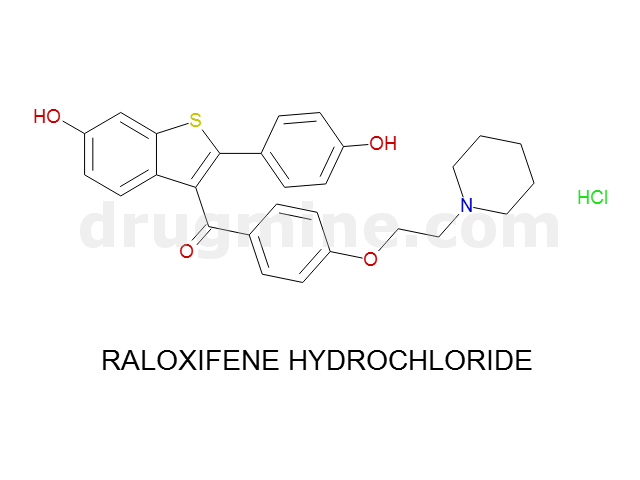
Name: RALOXIFENE HYDROCHLORIDE
ID :
MW: 474
Number of atoms: 34
Molecular_Formula: C28H27NO4S
Alogp: 6.461
Indication class : Anti-Estrogen
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
raloxifene hydrochloride containing products summary
There are in total 4 different products containing the active ingredient raloxifene hydrochloride. From the 4 drug products, zero have been discontinued.Product id = 21123
Application Number = 20815
Date of Application = 9,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = EVISTA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LILLY
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 60MG
----
Product id = 27285
Application Number = 78193
Date of Application = 4,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = RALOXIFENE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 27284
Application Number = 90842
Date of Application = 24,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = RALOXIFENE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 27286
Application Number = 200825
Date of Application = 21,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = RALOXIFENE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----