rabeprazole-sodium
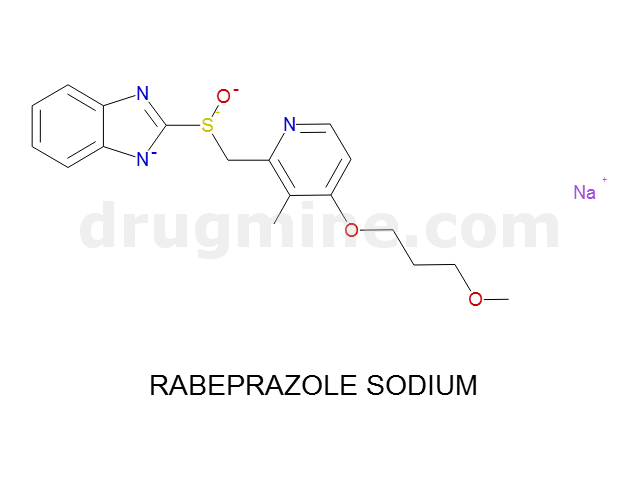
Name: RABEPRAZOLE SODIUM
ID :
MW: 358
Number of atoms: 25
Molecular_Formula: C18H20N3O3S
Alogp: 2.829
Indication class : Anti-Ulcerative; Gastric Acid Pump Inhibitor
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
rabeprazole sodium containing products summary
There are in total 10 different products containing the active ingredient rabeprazole sodium. From the 10 drug products, 1 have been discontinued.Product id = 15388
Application Number = 20973
Date of Application = 29,, May, 2002
RX/OTC/DISCN = DISCN
Tradename = ACIPHEX
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = EISAI INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 15389
Application Number = 20973
Date of Application = 19,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = ACIPHEX
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = EISAI INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 20MG
----
Product id = 15594
Application Number = 76822
Date of Application = 8,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RABEPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 15590
Application Number = 76824
Date of Application = 8,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RABEPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 15593
Application Number = 76885
Date of Application = 8,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RABEPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 15592
Application Number = 78964
Date of Application = 8,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RABEPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 15591
Application Number = 90678
Date of Application = 8,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RABEPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = KREMERS URBAN DEV
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 15595
Application Number = 202376
Date of Application = 8,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = RABEPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 247
Application Number = 204736
Date of Application = 26,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = ACIPHEX SPRINKLE
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = N
Applicant Name = EISAI INC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 248
Application Number = 204736
Date of Application = 26,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = ACIPHEX SPRINKLE
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = N
Applicant Name = EISAI INC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 10MG
----