propafenone-hydrochloride
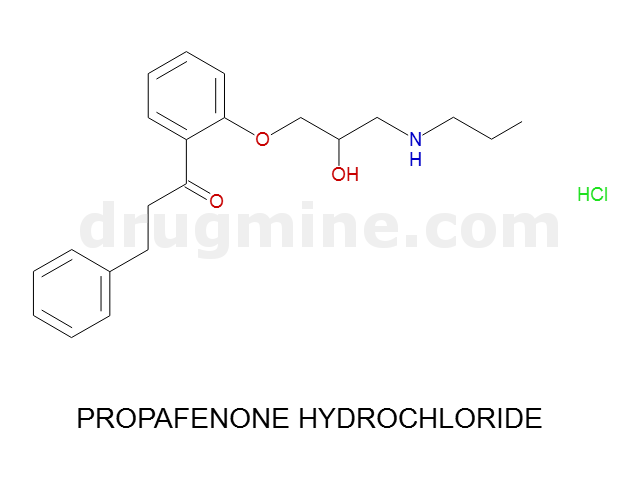
Name: PROPAFENONE HYDROCHLORIDE
ID :
MW: 341
Number of atoms: 25
Molecular_Formula: C21H27NO3
Alogp: 3.673
Indication class : Cardiac Depressant (anti-arrhythmic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
propafenone hydrochloride containing products summary
There are in total 23 different products containing the active ingredient propafenone hydrochloride. From the 23 drug products, 3 have been discontinued.Product id = 27813
Application Number = 19151
Date of Application = 27,, Nov, 1989
RX/OTC/DISCN = RX
Tradename = RYTHMOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 27815
Application Number = 19151
Date of Application = 27,, Nov, 1989
RX/OTC/DISCN = RX
Tradename = RYTHMOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 300MG
----
Product id = 27814
Application Number = 19151
Date of Application = 20,, Nov, 1992
RX/OTC/DISCN = RX
Tradename = RYTHMOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 225MG
----
Product id = 739
Application Number = 21416
Date of Application = 4,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = RYTHMOL SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 225MG
----
Product id = 740
Application Number = 21416
Date of Application = 4,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = RYTHMOL SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 325MG
----
Product id = 741
Application Number = 21416
Date of Application = 4,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = RYTHMOL SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 425MG
----
Product id = 26838
Application Number = 75203
Date of Application = 24,, Oct, 2000
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 26839
Application Number = 75203
Date of Application = 24,, Oct, 2000
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 225MG
----
Product id = 26835
Application Number = 75938
Date of Application = 17,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 26836
Application Number = 75938
Date of Application = 17,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 225MG
----
Product id = 26837
Application Number = 75938
Date of Application = 17,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 26829
Application Number = 75998
Date of Application = 29,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 26830
Application Number = 75998
Date of Application = 29,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 225MG
----
Product id = 26831
Application Number = 75998
Date of Application = 29,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 26832
Application Number = 76193
Date of Application = 7,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 26833
Application Number = 76193
Date of Application = 7,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 225MG
----
Product id = 26834
Application Number = 76193
Date of Application = 7,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 300MG
----
Product id = 26826
Application Number = 76550
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 26827
Application Number = 76550
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 225MG
----
Product id = 26828
Application Number = 76550
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 683
Application Number = 78540
Date of Application = 18,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 225MG
----
Product id = 684
Application Number = 78540
Date of Application = 18,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 325MG
----
Product id = 685
Application Number = 78540
Date of Application = 18,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = PROPAFENONE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 425MG
----