prednisolone-acetate
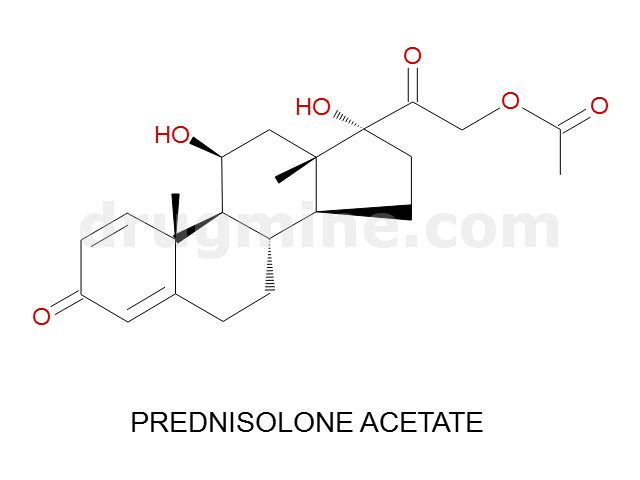

Name: PREDNISOLONE ACETATE
ID :
MW: 402
Number of atoms: 29
Molecular_Formula: C23H30O6
Alogp: 1.639
Indication class : Glucocorticoid
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
prednisolone acetate containing products summary
There are in total 37 different products containing the active ingredient prednisolone acetate. From the 37 drug products, 29 have been discontinued.Product id = 14563
Application Number = 10210
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = METIMYD
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;10%
----
Product id = 12346
Application Number = 10210
Date of Application = 9,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = METIMYD
Route/format = OPHTHALMIC / OINTMENT
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5%;10%
----
Product id = 9263
Application Number = 10255
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = METICORTELONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 10876
Application Number = 11446
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STERANE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 14627
Application Number = 12813
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = BLEPHAMIDE
Route/format = OPHTHALMIC / SUSPENSION
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 002
Tecode =
Rld = Yes
Strength = 0.2%;10%
----
Product id = 14573
Application Number = 17011
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PRED FORTE
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1%
----
Product id = 14574
Application Number = 17100
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PRED MILD
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.12%
----
Product id = 14550
Application Number = 17468
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ECONOPRED
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode =
Rld = No
Strength = 0.125%
----
Product id = 14571
Application Number = 17469
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = OMNIPRED
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALCON
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1%
----
Product id = 14697
Application Number = 22067
Date of Application = 17,, Jan, 2008
RX/OTC/DISCN = DISCN
Tradename = FLO-PRED
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 14698
Application Number = 22067
Date of Application = 17,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = FLO-PRED
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = TARO
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 15MG BASE/5ML
----
Product id = 14572
Application Number = 50081
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = POLY-PRED
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.35% BASE;10,000 UNITS/ML;0.5%
----
Product id = 14575
Application Number = 50586
Date of Application = 10,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = PRED-G
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.3% BASE;1%
----
Product id = 12367
Application Number = 50612
Date of Application = 1,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = PRED-G
Route/format = OPHTHALMIC / OINTMENT
Application Type = N
Applicant Name = ALLERGAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.3% BASE;0.6%
----
Product id = 14566
Application Number = 61037
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-DELTA-CORTEF
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/ML;0.25%
----
Product id = 12351
Application Number = 61039
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-DELTA-CORTEF
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.5%
----
Product id = 12350
Application Number = 61039
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NEO-DELTA-CORTEF
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 3.5MG BASE/GM;0.25%
----
Product id = 10412
Application Number = 83032
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 10418
Application Number = 83398
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 10419
Application Number = 83654
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 10414
Application Number = 83738
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEL MAR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 10415
Application Number = 83738
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEL MAR
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10421
Application Number = 83764
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10420
Application Number = 83767
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG/ML
----
Product id = 10413
Application Number = 84492
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10416
Application Number = 84717
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG/ML
----
Product id = 10417
Application Number = 84717
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CENT PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10422
Application Number = 85781
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PREDNISOLONE ACETATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 14629
Application Number = 87547
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ISOPTO CETAPRED
Route/format = OPHTHALMIC / SUSPENSION
Application Type = A
Applicant Name = ALCON
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25%;10%
----
Product id = 12312
Application Number = 87748
Date of Application = 3,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = BLEPHAMIDE S.O.P.
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = ALLERGAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.2%;10%
----
Product id = 12314
Application Number = 87771
Date of Application = 6,, Aug, 1993
RX/OTC/DISCN = DISCN
Tradename = CETAPRED
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = ALCON
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25%;10%
----
Product id = 14577
Application Number = 88007
Date of Application = 19,, Apr, 1983
RX/OTC/DISCN = DISCN
Tradename = PREDSULFAIR
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;10%
----
Product id = 12368
Application Number = 88032
Date of Application = 15,, Apr, 1983
RX/OTC/DISCN = DISCN
Tradename = PREDSULFAIR
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;10%
----
Product id = 14576
Application Number = 88059
Date of Application = 29,, Jul, 1983
RX/OTC/DISCN = DISCN
Tradename = PREDAMIDE
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;10%
----
Product id = 14580
Application Number = 88089
Date of Application = 28,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = SULPHRIN
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;10%
----
Product id = 12377
Application Number = 88791
Date of Application = 5,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = VASOCIDIN
Route/format = OPHTHALMIC / OINTMENT
Application Type = A
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5%;10%
----
Product id = 14578
Application Number = 88837
Date of Application = 24,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = PREDSULFAIR II
Route/format = OPHTHALMIC / SUSPENSION/DROPS
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.2%;10%
----