prazosin-hydrochloride
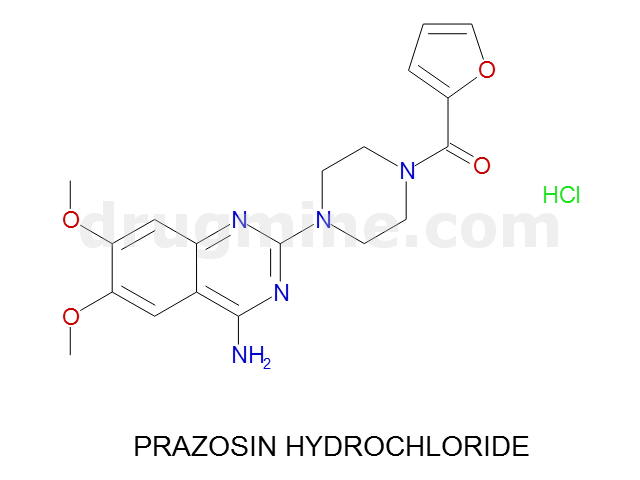
Name: PRAZOSIN HYDROCHLORIDE
ID :
MW: 383
Number of atoms: 28
Molecular_Formula: C19H21N5O4
Alogp: 2.107
Indication class : Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
prazosin hydrochloride containing products summary
There are in total 29 different products containing the active ingredient prazosin hydrochloride. From the 29 drug products, 20 have been discontinued.Product id = 2511
Application Number = 17442
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MINIPRESS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 2509
Application Number = 17442
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MINIPRESS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2510
Application Number = 17442
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = MINIPRESS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 2MG BASE
----
Product id = 2512
Application Number = 17986
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MINIZIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG;EQ 1MG BASE
----
Product id = 2513
Application Number = 17986
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MINIZIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG;EQ 2MG BASE
----
Product id = 2514
Application Number = 17986
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MINIZIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode =
Rld = No
Strength = 0.5MG;EQ 5MG BASE
----
Product id = 16243
Application Number = 19775
Date of Application = 29,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = MINIPRESS XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 16244
Application Number = 19775
Date of Application = 29,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = MINIPRESS XL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 2958
Application Number = 71745
Date of Application = 12,, Sep, 1988
RX/OTC/DISCN = RX
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 2947
Application Number = 71994
Date of Application = 12,, Sep, 1988
RX/OTC/DISCN = RX
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2957
Application Number = 71995
Date of Application = 12,, Sep, 1988
RX/OTC/DISCN = RX
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 2960
Application Number = 72333
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 2959
Application Number = 72352
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2950
Application Number = 72575
Date of Application = 16,, May, 1989
RX/OTC/DISCN = RX
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 2949
Application Number = 72575
Date of Application = 16,, May, 1989
RX/OTC/DISCN = RX
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 2948
Application Number = 72575
Date of Application = 16,, May, 1989
RX/OTC/DISCN = RX
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2954
Application Number = 72576
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2955
Application Number = 72577
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 2956
Application Number = 72578
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 2961
Application Number = 72609
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 2944
Application Number = 72705
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2945
Application Number = 72706
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 2946
Application Number = 72707
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 2941
Application Number = 72782
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2942
Application Number = 72783
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 2943
Application Number = 72784
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 2952
Application Number = 72921
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 2951
Application Number = 72991
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE
----
Product id = 2953
Application Number = 72992
Date of Application = 16,, May, 1989
RX/OTC/DISCN = DISCN
Tradename = PRAZOSIN HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----