pravastatin-sodium
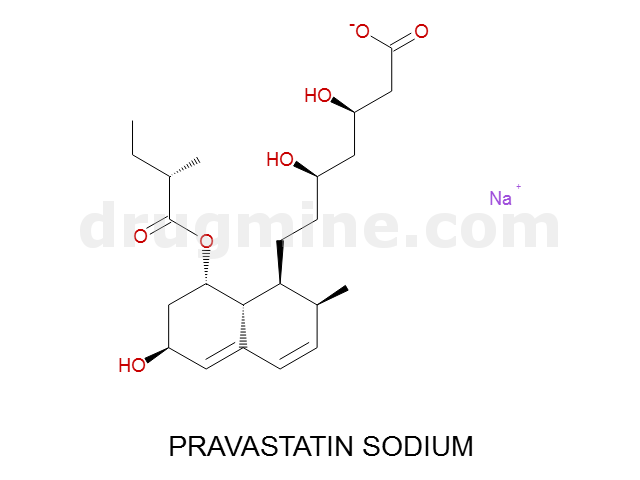
Name: PRAVASTATIN SODIUM
ID :
MW: 424
Number of atoms: 30
Molecular_Formula: C23H35O7
Alogp: 0.682
Indication class : Antihyperlipidemic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pravastatin sodium containing products summary
There are in total 61 different products containing the active ingredient pravastatin sodium. From the 61 drug products, 15 have been discontinued.Product id = 26431
Application Number = 19898
Date of Application = 31,, Oct, 1991
RX/OTC/DISCN = DISCN
Tradename = PRAVACHOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26432
Application Number = 19898
Date of Application = 31,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = PRAVACHOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26433
Application Number = 19898
Date of Application = 22,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = PRAVACHOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26434
Application Number = 19898
Date of Application = 18,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = PRAVACHOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 008
Tecode = AB
Rld = Yes
Strength = 80MG
----
Product id = 17126
Application Number = 21387
Date of Application = 24,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = PRAVIGARD PAC (COPACKAGED)
Route/format = ORAL / TABLET, TABLET, TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 81MG,N/A;N/A,20MG
----
Product id = 17127
Application Number = 21387
Date of Application = 24,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = PRAVIGARD PAC (COPACKAGED)
Route/format = ORAL / TABLET, TABLET, TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 81MG,N/A;N/A,40MG
----
Product id = 17128
Application Number = 21387
Date of Application = 24,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = PRAVIGARD PAC (COPACKAGED)
Route/format = ORAL / TABLET, TABLET, TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 81MG,N/A;N/A,80MG
----
Product id = 17129
Application Number = 21387
Date of Application = 24,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = PRAVIGARD PAC (COPACKAGED)
Route/format = ORAL / TABLET, TABLET, TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode =
Rld = No
Strength = 325MG,N/A;N/A,20MG
----
Product id = 17130
Application Number = 21387
Date of Application = 24,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = PRAVIGARD PAC (COPACKAGED)
Route/format = ORAL / TABLET, TABLET, TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 005
Tecode =
Rld = No
Strength = 325MG,N/A;N/A,40MG
----
Product id = 17132
Application Number = 21387
Date of Application = 24,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = PRAVIGARD PAC (COPACKAGED)
Route/format = ORAL / TABLET, TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 006
Tecode =
Rld = No
Strength = 325MG,N/A;N/A,80MG
----
Product id = 26474
Application Number = 76056
Date of Application = 24,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26475
Application Number = 76056
Date of Application = 24,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26476
Application Number = 76056
Date of Application = 24,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26435
Application Number = 76341
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26436
Application Number = 76341
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26437
Application Number = 76341
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26438
Application Number = 76341
Date of Application = 28,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26472
Application Number = 76397
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26471
Application Number = 76397
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26470
Application Number = 76397
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26466
Application Number = 76445
Date of Application = 23,, Apr, 2007
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26467
Application Number = 76445
Date of Application = 23,, Apr, 2007
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26468
Application Number = 76445
Date of Application = 23,, Apr, 2007
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 003
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26469
Application Number = 76445
Date of Application = 23,, Apr, 2007
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 004
Tecode =
Rld = No
Strength = 80MG
----
Product id = 26442
Application Number = 76714
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26443
Application Number = 76714
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26444
Application Number = 76714
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26445
Application Number = 76714
Date of Application = 28,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26481
Application Number = 76939
Date of Application = 28,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26480
Application Number = 76939
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26479
Application Number = 76939
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26478
Application Number = 76939
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26454
Application Number = 77013
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26455
Application Number = 77013
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26456
Application Number = 77013
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26457
Application Number = 77013
Date of Application = 28,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26473
Application Number = 77491
Date of Application = 11,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26462
Application Number = 77730
Date of Application = 21,, Nov, 2006
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26463
Application Number = 77730
Date of Application = 21,, Nov, 2006
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 26464
Application Number = 77730
Date of Application = 21,, Nov, 2006
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 003
Tecode =
Rld = No
Strength = 30MG
----
Product id = 26465
Application Number = 77730
Date of Application = 21,, Nov, 2006
RX/OTC/DISCN = DISCN
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 005
Tecode =
Rld = No
Strength = 40MG
----
Product id = 26482
Application Number = 77751
Date of Application = 30,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26483
Application Number = 77751
Date of Application = 30,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26484
Application Number = 77751
Date of Application = 30,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26485
Application Number = 77751
Date of Application = 30,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26477
Application Number = 77793
Date of Application = 15,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26439
Application Number = 77904
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26440
Application Number = 77904
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26441
Application Number = 77904
Date of Application = 23,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26450
Application Number = 77917
Date of Application = 8,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26451
Application Number = 77917
Date of Application = 8,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26452
Application Number = 77917
Date of Application = 8,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26453
Application Number = 77917
Date of Application = 8,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26446
Application Number = 77987
Date of Application = 11,, May, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26447
Application Number = 77987
Date of Application = 11,, May, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26448
Application Number = 77987
Date of Application = 11,, May, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26449
Application Number = 77987
Date of Application = 28,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 26458
Application Number = 79187
Date of Application = 27,, May, 2010
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26459
Application Number = 79187
Date of Application = 27,, May, 2010
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26460
Application Number = 79187
Date of Application = 27,, May, 2010
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26461
Application Number = 79187
Date of Application = 27,, May, 2010
RX/OTC/DISCN = RX
Tradename = PRAVASTATIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 80MG
----