pindolol
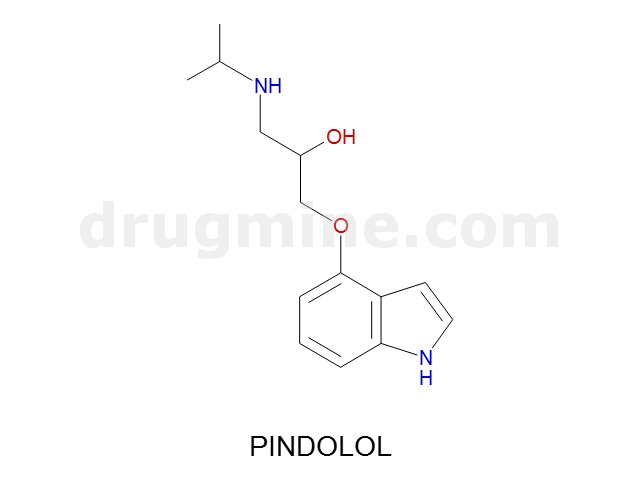
Name: PINDOLOL
ID :
MW: 248
Number of atoms: 18
Molecular_Formula: C14H20N2O2
Alogp: 1.925
Indication class : Vasodilator
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pindolol containing products summary
There are in total 24 different products containing the active ingredient pindolol. From the 24 drug products, 20 have been discontinued.Product id = 29603
Application Number = 18285
Date of Application = 3,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = VISKEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 29604
Application Number = 18285
Date of Application = 3,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = VISKEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 29601
Application Number = 18872
Date of Application = 22,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = VISKAZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;5MG
----
Product id = 29602
Application Number = 18872
Date of Application = 22,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = VISKAZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;10MG
----
Product id = 26252
Application Number = 73608
Date of Application = 29,, Mar, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26253
Application Number = 73609
Date of Application = 29,, Mar, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26254
Application Number = 73661
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26256
Application Number = 73661
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26240
Application Number = 73687
Date of Application = 26,, Feb, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26241
Application Number = 73687
Date of Application = 26,, Feb, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26244
Application Number = 74013
Date of Application = 24,, Sep, 1992
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26246
Application Number = 74018
Date of Application = 24,, Sep, 1992
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26245
Application Number = 74019
Date of Application = 3,, Sep, 1992
RX/OTC/DISCN = RX
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 26247
Application Number = 74019
Date of Application = 3,, Sep, 1992
RX/OTC/DISCN = RX
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 26242
Application Number = 74063
Date of Application = 27,, Jan, 1994
RX/OTC/DISCN = RX
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 26243
Application Number = 74063
Date of Application = 27,, Jan, 1994
RX/OTC/DISCN = RX
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26255
Application Number = 74123
Date of Application = 17,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26257
Application Number = 74123
Date of Application = 17,, Apr, 1997
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26250
Application Number = 74125
Date of Application = 28,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26251
Application Number = 74125
Date of Application = 28,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26258
Application Number = 74437
Date of Application = 27,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26259
Application Number = 74437
Date of Application = 27,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 26248
Application Number = 74474
Date of Application = 28,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26249
Application Number = 74474
Date of Application = 28,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = PINDOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----