phenytoin
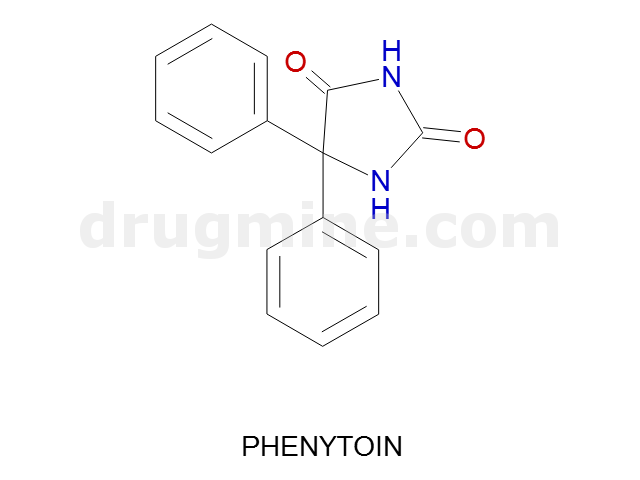

Name: PHENYTOIN
ID :
MW: 252
Number of atoms: 19
Molecular_Formula: C15H12N2O2
Alogp: 2.105
Indication class : Anticonvulsant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
phenytoin containing products summary
There are in total 11 different products containing the active ingredient phenytoin. From the 11 drug products, 2 have been discontinued.Product id = 14671
Application Number = 8762
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DILANTIN-125
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 125MG/5ML
----
Product id = 14672
Application Number = 8762
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DILANTIN-30
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = PARKE DAVIS
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG/5ML
----
Product id = 14787
Application Number = 40342
Date of Application = 31,, Jan, 2001
RX/OTC/DISCN = RX
Tradename = PHENYTOIN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = VISTAPHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG/5ML
----
Product id = 14789
Application Number = 40420
Date of Application = 19,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = PHENYTOIN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG/5ML
----
Product id = 14786
Application Number = 40521
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = PHENYTOIN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG/5ML
----
Product id = 14788
Application Number = 40610
Date of Application = 18,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = PHENYTOIN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = VISTAPHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 125MG/5ML
----
Product id = 15352
Application Number = 40884
Date of Application = 28,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = PHENYTOIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 15248
Application Number = 84427
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DILANTIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = PFIZER PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 50MG
----
Product id = 14785
Application Number = 89892
Date of Application = 25,, Sep, 1992
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG/5ML
----
Product id = 15354
Application Number = 200565
Date of Application = 17,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = PHENYTOIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 15353
Application Number = 200691
Date of Application = 26,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = PHENYTOIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----