pentoxifylline
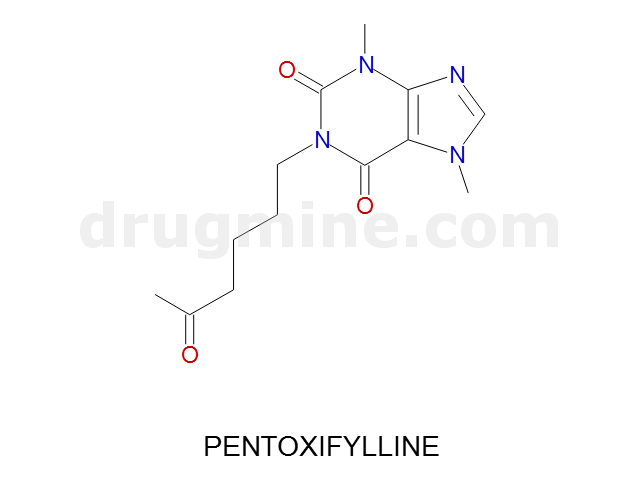

Name: PENTOXIFYLLINE
ID :
MW: 278
Number of atoms: 20
Molecular_Formula: C13H18N4O3
Alogp: 0.507
Indication class : Vasodilator
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pentoxifylline containing products summary
There are in total 11 different products containing the active ingredient pentoxifylline. From the 11 drug products, 5 have been discontinued.Product id = 16728
Application Number = 18631
Date of Application = 30,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = TRENTAL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 16486
Application Number = 74425
Date of Application = 8,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 16487
Application Number = 74874
Date of Application = 25,, May, 1999
RX/OTC/DISCN = RX
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 16484
Application Number = 74877
Date of Application = 8,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 16482
Application Number = 74878
Date of Application = 9,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 16491
Application Number = 74962
Date of Application = 31,, Mar, 1999
RX/OTC/DISCN = RX
Tradename = PENTOXIL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 16489
Application Number = 75028
Date of Application = 20,, Jul, 1998
RX/OTC/DISCN = RX
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = VALEANT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 16485
Application Number = 75093
Date of Application = 10,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 16490
Application Number = 75107
Date of Application = 4,, Sep, 1998
RX/OTC/DISCN = DISCN
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----
Product id = 16483
Application Number = 75191
Date of Application = 9,, Jun, 1999
RX/OTC/DISCN = RX
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 16488
Application Number = 75199
Date of Application = 3,, Sep, 1999
RX/OTC/DISCN = DISCN
Tradename = PENTOXIFYLLINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 400MG
----