pantoprazole-sodium
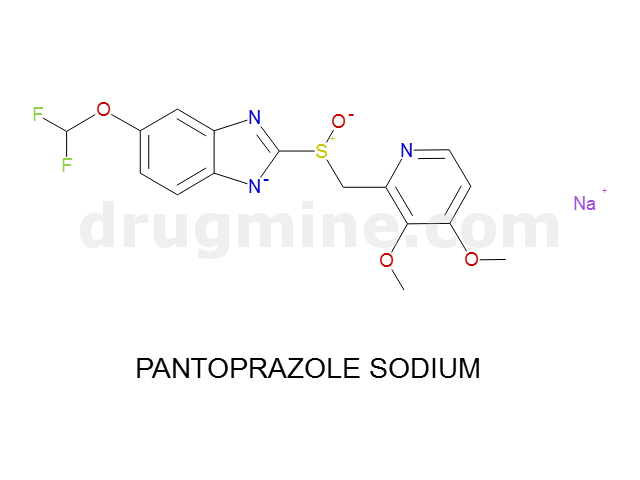
Name: PANTOPRAZOLE SODIUM
ID :
MW: 382
Number of atoms: 26
Molecular_Formula: C16H14F2N3O4S
Alogp: 3.201
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
pantoprazole sodium containing products summary
There are in total 36 different products containing the active ingredient pantoprazole sodium. From the 36 drug products, zero have been discontinued.Product id = 15588
Application Number = 20987
Date of Application = 2,, Feb, 2000
RX/OTC/DISCN = RX
Tradename = PROTONIX
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 40MG BASE
----
Product id = 15587
Application Number = 20987
Date of Application = 12,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = PROTONIX
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 11886
Application Number = 20988
Date of Application = 22,, Mar, 2001
RX/OTC/DISCN = RX
Tradename = PROTONIX IV
Route/format = IV (INFUSION) / INJECTABLE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 40MG BASE/VIAL
----
Product id = 4972
Application Number = 22020
Date of Application = 14,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = PROTONIX
Route/format = ORAL / FOR SUSPENSION, DELAYED RELEASE
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 40MG BASE
----
Product id = 15575
Application Number = 77056
Date of Application = 2,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15576
Application Number = 77056
Date of Application = 2,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15573
Application Number = 77058
Date of Application = 10,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15574
Application Number = 77058
Date of Application = 10,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = SUN PHARMA GLOBAL
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15556
Application Number = 77619
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15557
Application Number = 77619
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15562
Application Number = 78281
Date of Application = 20,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15563
Application Number = 78281
Date of Application = 20,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 11880
Application Number = 79197
Date of Application = 8,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = IV (INFUSION) / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 40MG BASE/VIAL
----
Product id = 15577
Application Number = 90074
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15578
Application Number = 90074
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15550
Application Number = 90797
Date of Application = 7,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS TOTOWA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15551
Application Number = 90797
Date of Application = 7,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ACTAVIS TOTOWA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15552
Application Number = 90807
Date of Application = 2,, May, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15553
Application Number = 90807
Date of Application = 2,, May, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15560
Application Number = 90901
Date of Application = 30,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15561
Application Number = 90901
Date of Application = 30,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15566
Application Number = 90970
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15567
Application Number = 90970
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15579
Application Number = 91231
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15580
Application Number = 91231
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15571
Application Number = 200794
Date of Application = 2,, May, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15572
Application Number = 200794
Date of Application = 2,, May, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15564
Application Number = 200821
Date of Application = 16,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15565
Application Number = 200821
Date of Application = 16,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15554
Application Number = 202038
Date of Application = 28,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15555
Application Number = 202038
Date of Application = 28,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15568
Application Number = 202052
Date of Application = 2,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15569
Application Number = 202052
Date of Application = 2,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15558
Application Number = 202882
Date of Application = 10,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = HETERO LABS LTD V
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 15559
Application Number = 202882
Date of Application = 10,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = HETERO LABS LTD V
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 15570
Application Number = 203024
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = PANTOPRAZOLE SODIUM
Route/format = ORAL / TABLET, DELAYED RELEASE
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----