norgestimate
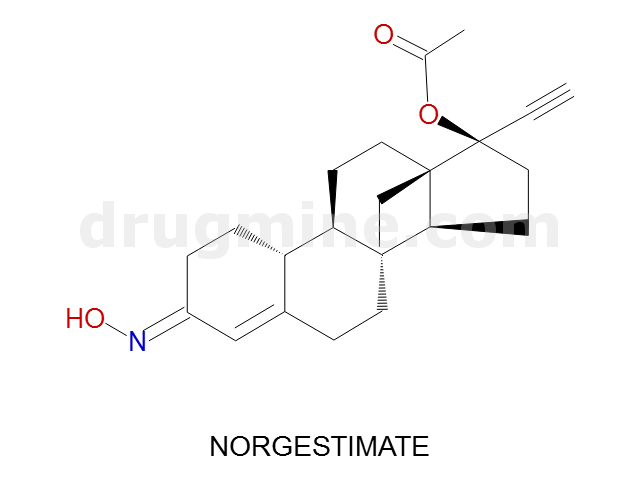
Name: NORGESTIMATE
ID :
MW: 369
Number of atoms: 27
Molecular_Formula: C23H31NO3
Alogp: 5.218
Indication class : Progestin
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
norgestimate containing products summary
There are in total 22 different products containing the active ingredient norgestimate. From the 22 drug products, 7 have been discontinued.Product id = 29952
Application Number = 19653
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ORTHO CYCLEN-21
Route/format = ORAL-21 / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.035MG;0.25MG
----
Product id = 30089
Application Number = 19653
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = ORTHO CYCLEN-28
Route/format = ORAL-28 / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 0.035MG;0.25MG
----
Product id = 30090
Application Number = 19697
Date of Application = 3,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = ORTHO TRI-CYCLEN
Route/format = ORAL-28 / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 29953
Application Number = 19697
Date of Application = 3,, Jul, 1992
RX/OTC/DISCN = DISCN
Tradename = ORTHO TRI-CYCLEN
Route/format = ORAL-21 / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 26643
Application Number = 21040
Date of Application = 22,, Oct, 1999
RX/OTC/DISCN = DISCN
Tradename = PREFEST
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG,1MG;N/A,0.09MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 30091
Application Number = 21241
Date of Application = 22,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = ORTHO TRI-CYCLEN LO
Route/format = ORAL-28 / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.025MG,0.025MG,0.025MG;0.18MG,0.215MG,0.25MG
----
Product id = 30116
Application Number = 75804
Date of Application = 25,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = SPRINTEC
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG;0.25MG
----
Product id = 30124
Application Number = 75808
Date of Application = 29,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = TRI-SPRINTEC
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 30115
Application Number = 76334
Date of Application = 9,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = PREVIFEM
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG;0.25MG
----
Product id = 30123
Application Number = 76335
Date of Application = 26,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = TRI-PREVIFEM
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 30075
Application Number = 76626
Date of Application = 17,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = NORGESTIMATE AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 30076
Application Number = 76627
Date of Application = 17,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = NORGESTIMATE AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.035MG;0.25MG
----
Product id = 30118
Application Number = 76784
Date of Application = 29,, Jun, 2009
RX/OTC/DISCN = DISCN
Tradename = TRI LO SPRINTEC
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025MG,0.025MG,0.025MG;0.18MG,0.215MG,0.25MG
----
Product id = 21019
Application Number = 76812
Date of Application = 29,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = ESTRADIOL AND NORGESTIMATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1MG,1MG;N/A,0.09MG
----
Product id = 30074
Application Number = 90479
Date of Application = 9,, Mar, 2011
RX/OTC/DISCN = DISCN
Tradename = NORGESTIMATE AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025MG,0.025MG,0.025MG;0.18MG,0.215MG,0.25MG
----
Product id = 30046
Application Number = 90523
Date of Application = 23,, May, 2012
RX/OTC/DISCN = RX
Tradename = MONO-LINYAH
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = NOVAST LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG;0.25MG
----
Product id = 30121
Application Number = 90524
Date of Application = 30,, May, 2012
RX/OTC/DISCN = RX
Tradename = TRI-LINYAH
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = NOVAST LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 30119
Application Number = 90793
Date of Application = 30,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = TRI-ESTARYLLA
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 30007
Application Number = 90794
Date of Application = 30,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = ESTARYLLA
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG;0.25MG
----
Product id = 25474
Application Number = 200494
Date of Application = 17,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = NORGESTIMATE AND ETHINYL ESTRADIOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG,0.035MG,0.035MG;0.18MG,0.215MG,0.25MG
----
Product id = 30072
Application Number = 200538
Date of Application = 5,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = NORGESTIMATE AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.035MG;0.25MG
----
Product id = 30073
Application Number = 200541
Date of Application = 25,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = NORGESTIMATE AND ETHINYL ESTRADIOL
Route/format = ORAL-28 / TABLET
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.025MG,0.025MG,0.025MG;0.18MG,0.215MG,0.25MG
----