nitroglycerin
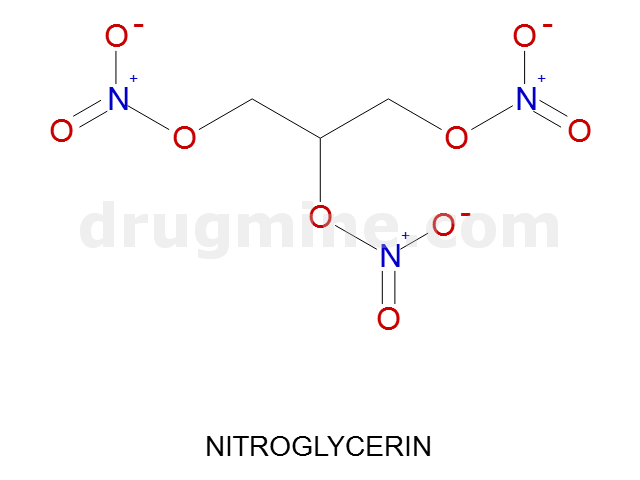

Name: NITROGLYCERIN
ID :
MW: 227
Number of atoms: 15
Molecular_Formula: C3H5N3O9
Alogp: 5.822
Indication class : Vasodilator (coronary)
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
nitroglycerin containing products summary
There are in total 64 different products containing the active ingredient nitroglycerin. From the 64 drug products, 32 have been discontinued.Product id = 9666
Application Number = 18531
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 11208
Application Number = 18537
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIDIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 11207
Application Number = 18537
Date of Application = 16,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = TRIDIL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG/ML
----
Product id = 9685
Application Number = 18588
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NITROSTAT
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.8MG/ML
----
Product id = 9687
Application Number = 18588
Date of Application = 23,, Dec, 1983
RX/OTC/DISCN = DISCN
Tradename = NITROSTAT
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PARKE DAVIS
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9662
Application Number = 18621
Date of Application = 5,, Jan, 1982
RX/OTC/DISCN = DISCN
Tradename = NITRO-BID
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9680
Application Number = 18672
Date of Application = 30,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = NITRONAL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = POHL BOSKAMP
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9661
Application Number = 18672
Date of Application = 30,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = NITRO IV
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = POHL BOSKAMP
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 98
Application Number = 18705
Date of Application = 31,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = NITROLINGUAL
Route/format = SUBLINGUAL / AEROSOL
Application Type = N
Applicant Name = POHL BOSKAMP
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/SPRAY
----
Product id = 14406
Application Number = 18705
Date of Application = 10,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = NITROLINGUAL PUMPSPRAY
Route/format = SUBLINGUAL / SPRAY, METERED
Application Type = N
Applicant Name = POHL BOSKAMP
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 0.4MG/SPRAY
----
Product id = 9679
Application Number = 18774
Date of Application = 19,, Jan, 1983
RX/OTC/DISCN = DISCN
Tradename = NITROL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = RORER
ProductNo = 001
Tecode =
Rld = No
Strength = 0.8MG/ML
----
Product id = 9672
Application Number = 19970
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 10MG/100ML
----
Product id = 9673
Application Number = 19970
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 20MG/100ML
----
Product id = 9674
Application Number = 19970
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 40MG/100ML
----
Product id = 4774
Application Number = 20144
Date of Application = 27,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = TRANSDERM-NITRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG/HR
----
Product id = 4775
Application Number = 20144
Date of Application = 27,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = TRANSDERM-NITRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.2MG/HR
----
Product id = 4776
Application Number = 20144
Date of Application = 27,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = TRANSDERM-NITRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = No
Strength = 0.4MG/HR
----
Product id = 4777
Application Number = 20144
Date of Application = 27,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = TRANSDERM-NITRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode =
Rld = No
Strength = 0.6MG/HR
----
Product id = 4778
Application Number = 20144
Date of Application = 27,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = TRANSDERM-NITRO
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 0.8MG/HR
----
Product id = 4743
Application Number = 20145
Date of Application = 4,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = NITRO-DUR
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB1
Rld = Yes
Strength = 0.1MG/HR
----
Product id = 4744
Application Number = 20145
Date of Application = 4,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = NITRO-DUR
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode = AB1
Rld = Yes
Strength = 0.2MG/HR
----
Product id = 4745
Application Number = 20145
Date of Application = 4,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = NITRO-DUR
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 003
Tecode =
Rld = Yes
Strength = 0.3MG/HR
----
Product id = 4746
Application Number = 20145
Date of Application = 4,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = NITRO-DUR
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 004
Tecode = AB1
Rld = Yes
Strength = 0.4MG/HR
----
Product id = 4747
Application Number = 20145
Date of Application = 4,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = NITRO-DUR
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 005
Tecode = AB1
Rld = Yes
Strength = 0.6MG/HR
----
Product id = 4748
Application Number = 20145
Date of Application = 4,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = NITRO-DUR
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 006
Tecode =
Rld = Yes
Strength = 0.8MG/HR
----
Product id = 30206
Application Number = 21134
Date of Application = 1,, May, 2000
RX/OTC/DISCN = RX
Tradename = NITROSTAT
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.3MG
----
Product id = 30207
Application Number = 21134
Date of Application = 1,, May, 2000
RX/OTC/DISCN = RX
Tradename = NITROSTAT
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.4MG
----
Product id = 30208
Application Number = 21134
Date of Application = 1,, May, 2000
RX/OTC/DISCN = RX
Tradename = NITROSTAT
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 003
Tecode =
Rld = Yes
Strength = 0.6MG
----
Product id = 12294
Application Number = 21359
Date of Application = 21,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = RECTIV
Route/format = INTRA-ANAL / OINTMENT
Application Type = N
Applicant Name = FOREST LABS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.4%
----
Product id = 91
Application Number = 21780
Date of Application = 2,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = NITROMIST
Route/format = SUBLINGUAL / AEROSOL, METERED
Application Type = N
Applicant Name = MIST PHARMS LLC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.4MG/SPRAY
----
Product id = 9667
Application Number = 70026
Date of Application = 10,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9664
Application Number = 70077
Date of Application = 13,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9670
Application Number = 70633
Date of Application = 19,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9671
Application Number = 70634
Date of Application = 19,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9686
Application Number = 70863
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = NITROSTAT
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9688
Application Number = 70871
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = NITROSTAT
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9689
Application Number = 70872
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = NITROSTAT
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9663
Application Number = 71159
Date of Application = 28,, Feb, 1990
RX/OTC/DISCN = DISCN
Tradename = NITRO-BID
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 9665
Application Number = 71203
Date of Application = 8,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9668
Application Number = 71492
Date of Application = 24,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 9676
Application Number = 71846
Date of Application = 31,, Aug, 1990
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/100ML
----
Product id = 9677
Application Number = 71847
Date of Application = 31,, Aug, 1990
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/100ML
----
Product id = 9678
Application Number = 71848
Date of Application = 31,, Aug, 1990
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 40MG/100ML
----
Product id = 9669
Application Number = 72034
Date of Application = 24,, May, 1988
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MG/ML
----
Product id = 9675
Application Number = 74083
Date of Application = 26,, Oct, 1994
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.1MG/ML
----
Product id = 4760
Application Number = 74559
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 001
Tecode = AB2
Rld = Yes
Strength = 0.6MG/HR
----
Product id = 4758
Application Number = 74559
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 002
Tecode = AB2
Rld = Yes
Strength = 0.4MG/HR
----
Product id = 4756
Application Number = 74559
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 003
Tecode = AB2
Rld = Yes
Strength = 0.2MG/HR
----
Product id = 4754
Application Number = 74559
Date of Application = 6,, Feb, 1998
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 004
Tecode =
Rld = Yes
Strength = 0.1MG/HR
----
Product id = 4761
Application Number = 74992
Date of Application = 12,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 001
Tecode =
Rld = No
Strength = 0.6MG/HR
----
Product id = 4759
Application Number = 74992
Date of Application = 12,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 002
Tecode =
Rld = No
Strength = 0.4MG/HR
----
Product id = 4757
Application Number = 74992
Date of Application = 12,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 003
Tecode =
Rld = No
Strength = 0.2MG/HR
----
Product id = 4755
Application Number = 74992
Date of Application = 12,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 004
Tecode =
Rld = No
Strength = 0.1MG/HR
----
Product id = 4752
Application Number = 75115
Date of Application = 10,, Aug, 2004
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = KREMERS URBAN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.2MG/HR
----
Product id = 4753
Application Number = 75115
Date of Application = 10,, Aug, 2004
RX/OTC/DISCN = DISCN
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = KREMERS URBAN PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.4MG/HR
----
Product id = 12616
Application Number = 87355
Date of Application = 8,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2%
----
Product id = 4723
Application Number = 89771
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = MINITRAN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = VALEANT PHARMS
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.1MG/HR
----
Product id = 4721
Application Number = 89772
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = MINITRAN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MEDICIS
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.2MG/HR
----
Product id = 4722
Application Number = 89773
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = MINITRAN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MEDICIS
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.4MG/HR
----
Product id = 4724
Application Number = 89774
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = MINITRAN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = VALEANT PHARMS
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.6MG/HR
----
Product id = 4749
Application Number = 89884
Date of Application = 30,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = HERCON PHARM
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 0.2MG/HR
----
Product id = 4750
Application Number = 89885
Date of Application = 30,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = HERCON PHARM
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 0.4MG/HR
----
Product id = 4751
Application Number = 89886
Date of Application = 30,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = HERCON PHARM
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 0.6MG/HR
----
Product id = 14405
Application Number = 91496
Date of Application = 20,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = NITROGLYCERIN
Route/format = SUBLINGUAL / SPRAY, METERED
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.4MG/SPRAY
----