nisoldipine
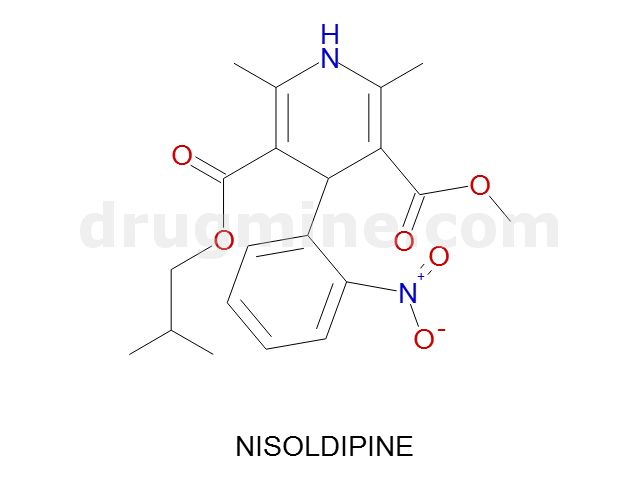
Name: NISOLDIPINE
ID :
MW: 388
Number of atoms: 28
Molecular_Formula: C20H24N2O6
Alogp: 2.958
Indication class : Vasodilator (coronary)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
nisoldipine containing products summary
There are in total 15 different products containing the active ingredient nisoldipine. From the 15 drug products, 5 have been discontinued.Product id = 16645
Application Number = 20356
Date of Application = 2,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16647
Application Number = 20356
Date of Application = 2,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16649
Application Number = 20356
Date of Application = 2,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 003
Tecode =
Rld = No
Strength = 30MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16651
Application Number = 20356
Date of Application = 2,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 004
Tecode =
Rld = No
Strength = 40MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16650
Application Number = 20356
Date of Application = 2,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 34MG
----
Product id = 16648
Application Number = 20356
Date of Application = 2,, Jan, 2008
RX/OTC/DISCN = DISCN
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 006
Tecode =
Rld = No
Strength = 25.5MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16646
Application Number = 20356
Date of Application = 2,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 007
Tecode = AB
Rld = Yes
Strength = 17MG
----
Product id = 16644
Application Number = 20356
Date of Application = 2,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = SULAR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIONOGI INC
ProductNo = 008
Tecode = AB
Rld = Yes
Strength = 8.5MG
----
Product id = 16377
Application Number = 79051
Date of Application = 25,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = NISOLDIPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 16379
Application Number = 79051
Date of Application = 25,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = NISOLDIPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = Yes
Strength = 30MG
----
Product id = 16381
Application Number = 79051
Date of Application = 25,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = NISOLDIPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = Yes
Strength = 40MG
----
Product id = 16375
Application Number = 91001
Date of Application = 26,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = NISOLDIPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8.5MG
----
Product id = 16376
Application Number = 91001
Date of Application = 26,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = NISOLDIPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 17MG
----
Product id = 16378
Application Number = 91001
Date of Application = 26,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = NISOLDIPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = No
Strength = 25.5MG
----
Product id = 16380
Application Number = 91001
Date of Application = 26,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = NISOLDIPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 34MG
----