nafcillin-sodium
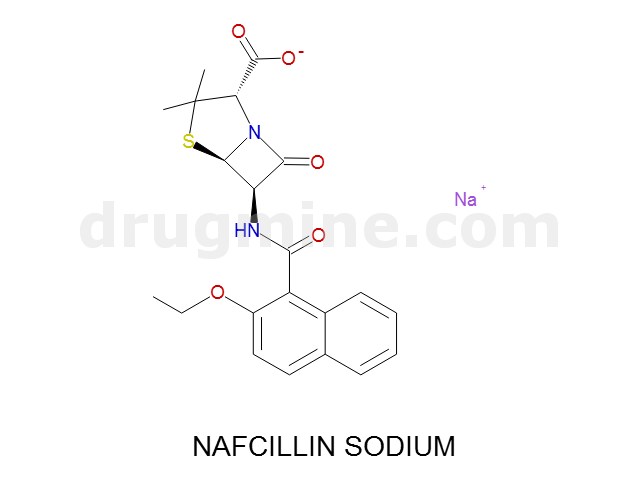
Name: NAFCILLIN SODIUM
ID :
MW: 413
Number of atoms: 29
Molecular_Formula: C21H21N2O5S
Alogp: 0.856
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
nafcillin sodium containing products summary
There are in total 49 different products containing the active ingredient nafcillin sodium. From the 49 drug products, 29 have been discontinued.Product id = 3678
Application Number = 50111
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 4937
Application Number = 50199
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/5ML
----
Product id = 11259
Application Number = 50320
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11260
Application Number = 50320
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11254
Application Number = 50320
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 11255
Application Number = 50320
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 4GM BASE/VIAL
----
Product id = 11256
Application Number = 50320
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 11257
Application Number = 50320
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 29284
Application Number = 50462
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 9537
Application Number = 50655
Date of Application = 31,, Oct, 1989
RX/OTC/DISCN = RX
Tradename = NALLPEN IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 20MG BASE/ML
----
Product id = 9536
Application Number = 50655
Date of Application = 31,, Oct, 1989
RX/OTC/DISCN = RX
Tradename = NALLPEN IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 2GM BASE/100ML
----
Product id = 9492
Application Number = 61984
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9489
Application Number = 61984
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9490
Application Number = 61984
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9491
Application Number = 61984
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 4GM BASE/VIAL
----
Product id = 9535
Application Number = 61999
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NALLPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9530
Application Number = 61999
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NALLPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9532
Application Number = 61999
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NALLPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9534
Application Number = 61999
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NALLPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9507
Application Number = 62527
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9502
Application Number = 62527
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 9504
Application Number = 62527
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 9506
Application Number = 62527
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = EQ 10GM BASE/VIAL
----
Product id = 11258
Application Number = 62717
Date of Application = 16,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11252
Application Number = 62717
Date of Application = 16,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11253
Application Number = 62717
Date of Application = 16,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = UNIPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9503
Application Number = 62732
Date of Application = 23,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 9505
Application Number = 62732
Date of Application = 23,, Dec, 1986
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 9531
Application Number = 62755
Date of Application = 19,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = NALLPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9533
Application Number = 62755
Date of Application = 19,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = NALLPEN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9513
Application Number = 62844
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 9508
Application Number = 62844
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9509
Application Number = 62844
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1.5GM BASE/VIAL
----
Product id = 9510
Application Number = 62844
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9511
Application Number = 62844
Date of Application = 26,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 4GM BASE/VIAL
----
Product id = 9512
Application Number = 63008
Date of Application = 29,, Sep, 1988
RX/OTC/DISCN = DISCN
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9496
Application Number = 90002
Date of Application = 30,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9497
Application Number = 90002
Date of Application = 30,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9498
Application Number = 90005
Date of Application = 20,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ISTITUTO BIO ITA SPA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9487
Application Number = 90560
Date of Application = 3,, Oct, 2011
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ANTIBIOTICE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9488
Application Number = 90560
Date of Application = 3,, Oct, 2011
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ANTIBIOTICE
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9501
Application Number = 90580
Date of Application = 24,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9499
Application Number = 90582
Date of Application = 24,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9500
Application Number = 90582
Date of Application = 24,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9493
Application Number = 91613
Date of Application = 26,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9494
Application Number = 91613
Date of Application = 26,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9495
Application Number = 91614
Date of Application = 26,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9485
Application Number = 200002
Date of Application = 7,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9486
Application Number = 200002
Date of Application = 7,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = NAFCILLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----