mitomycin
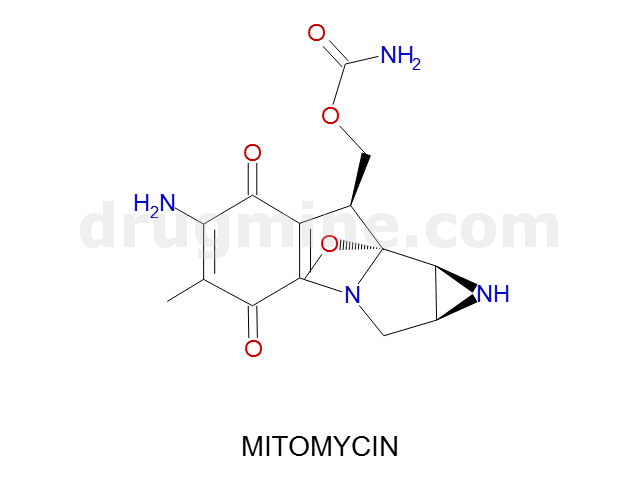
Name: MITOMYCIN
ID :
MW: 334
Number of atoms: 24
Molecular_Formula: C15H18N4O5
Alogp: -1.547
Indication class : Antineoplastic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
mitomycin containing products summary
There are in total 15 different products containing the active ingredient mitomycin. From the 15 drug products, 7 have been discontinued.Product id = 4960
Application Number = 22572
Date of Application = 7,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = MITOSOL
Route/format = TOPICAL / FOR SOLUTION
Application Type = N
Applicant Name = MOBIUS THERAP
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.2MG/VIAL
----
Product id = 9474
Application Number = 50450
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MUTAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BRISTOL
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/VIAL
----
Product id = 9475
Application Number = 50450
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MUTAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BRISTOL
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG/VIAL
----
Product id = 9435
Application Number = 50763
Date of Application = 14,, Nov, 2002
RX/OTC/DISCN = DISCN
Tradename = MITOZYTREX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SUPERGEN
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/VIAL
----
Product id = 9476
Application Number = 62336
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MUTAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG/VIAL
----
Product id = 9477
Application Number = 62336
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MUTAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG/VIAL
----
Product id = 9478
Application Number = 62336
Date of Application = 10,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = MUTAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS
ProductNo = 003
Tecode =
Rld = No
Strength = 40MG/VIAL
----
Product id = 9416
Application Number = 64106
Date of Application = 29,, Nov, 1995
RX/OTC/DISCN = DISCN
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG/VIAL
----
Product id = 9412
Application Number = 64117
Date of Application = 19,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5MG/VIAL
----
Product id = 9413
Application Number = 64117
Date of Application = 19,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode = AP
Rld = No
Strength = 20MG/VIAL
----
Product id = 9409
Application Number = 64144
Date of Application = 30,, Apr, 1998
RX/OTC/DISCN = RX
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 5MG/VIAL
----
Product id = 9410
Application Number = 64144
Date of Application = 30,, Apr, 1998
RX/OTC/DISCN = RX
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 20MG/VIAL
----
Product id = 9411
Application Number = 64144
Date of Application = 11,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 40MG/VIAL
----
Product id = 9414
Application Number = 64180
Date of Application = 23,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 5MG/VIAL
----
Product id = 9415
Application Number = 64180
Date of Application = 23,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = MITOMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode = AP
Rld = No
Strength = 20MG/VIAL
----