midodrine-hydrochloride
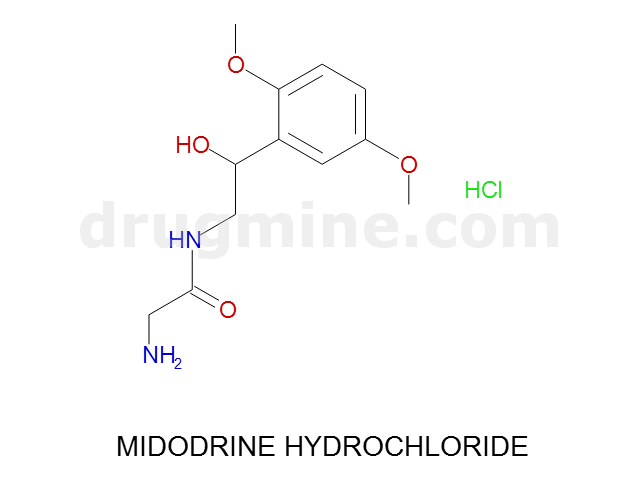

Name: MIDODRINE HYDROCHLORIDE
ID :
MW: 254
Number of atoms: 18
Molecular_Formula: C12H18N2O4
Alogp: -0.548
Indication class : Antihypotensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
midodrine hydrochloride containing products summary
There are in total 18 different products containing the active ingredient midodrine hydrochloride. From the 18 drug products, 3 have been discontinued.Product id = 26698
Application Number = 19815
Date of Application = 6,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = PROAMATINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 26699
Application Number = 19815
Date of Application = 6,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = PROAMATINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 26700
Application Number = 19815
Date of Application = 20,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = PROAMATINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24922
Application Number = 76449
Date of Application = 27,, May, 2004
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 24923
Application Number = 76449
Date of Application = 27,, May, 2004
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24924
Application Number = 76449
Date of Application = 16,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24928
Application Number = 76514
Date of Application = 11,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 24929
Application Number = 76514
Date of Application = 11,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24930
Application Number = 76514
Date of Application = 2,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24925
Application Number = 76577
Date of Application = 10,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 24926
Application Number = 76577
Date of Application = 10,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 24927
Application Number = 76577
Date of Application = 10,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 25679
Application Number = 76725
Date of Application = 3,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = ORVATEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 25680
Application Number = 76725
Date of Application = 3,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = ORVATEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 25681
Application Number = 76725
Date of Application = 3,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = ORVATEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24919
Application Number = 77746
Date of Application = 12,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 24920
Application Number = 77746
Date of Application = 12,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24921
Application Number = 77746
Date of Application = 12,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = MIDODRINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----