metronidazole
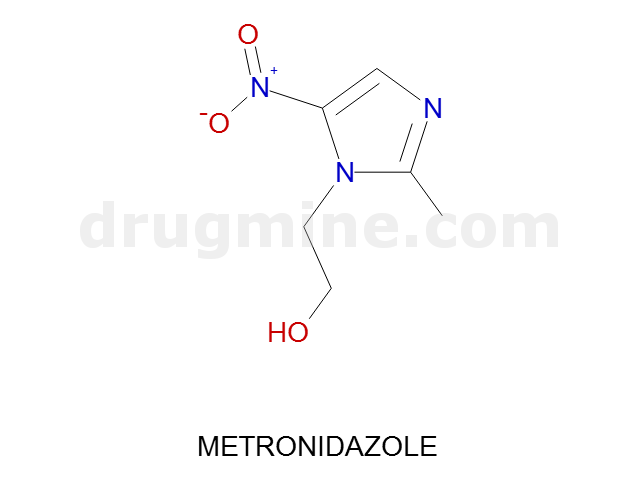
Name: METRONIDAZOLE
ID :
MW: 171
Number of atoms: 12
Molecular_Formula: C6H9N3O3
Alogp: -0.337
Indication class : Antiprotozoal (trichomonas)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
metronidazole containing products summary
There are in total 79 different products containing the active ingredient metronidazole. From the 79 drug products, 39 have been discontinued.Product id = 21332
Application Number = 12623
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FLAGYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 21333
Application Number = 12623
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FLAGYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 500MG
----
Product id = 7619
Application Number = 18353
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FLAGYL I.V. RTU IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 500MG/100ML
----
Product id = 24877
Application Number = 18517
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24878
Application Number = 18517
Date of Application = 5,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24899
Application Number = 18599
Date of Application = 17,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24901
Application Number = 18599
Date of Application = 13,, Feb, 1984
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24887
Application Number = 18620
Date of Application = 4,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24889
Application Number = 18620
Date of Application = 2,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 7618
Application Number = 18657
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = FLAGYL I.V. RTU IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 500MG/100ML
----
Product id = 9295
Application Number = 18674
Date of Application = 31,, Aug, 1982
RX/OTC/DISCN = DISCN
Tradename = METRO I.V.
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/100ML
----
Product id = 24888
Application Number = 18740
Date of Application = 22,, Oct, 1982
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24890
Application Number = 18740
Date of Application = 22,, Oct, 1982
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24900
Application Number = 18764
Date of Application = 17,, Sep, 1982
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24902
Application Number = 18764
Date of Application = 20,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24881
Application Number = 18818
Date of Application = 16,, Feb, 1983
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24883
Application Number = 18818
Date of Application = 16,, Feb, 1983
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24884
Application Number = 18845
Date of Application = 18,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 27046
Application Number = 18871
Date of Application = 2,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = PROTOSTAT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORTHO MCNEIL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 27047
Application Number = 18871
Date of Application = 2,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = PROTOSTAT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORTHO MCNEIL PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 9297
Application Number = 18889
Date of Application = 18,, Nov, 1983
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABBOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/100ML
----
Product id = 9305
Application Number = 18890
Date of Application = 18,, Nov, 1983
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 500MG/100ML
----
Product id = 9296
Application Number = 18900
Date of Application = 29,, Sep, 1983
RX/OTC/DISCN = RX
Tradename = METRO I.V. IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 500MG/100ML
----
Product id = 9299
Application Number = 18907
Date of Application = 30,, Mar, 1984
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/100ML
----
Product id = 24885
Application Number = 18930
Date of Application = 18,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24879
Application Number = 19029
Date of Application = 10,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = LNK
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 5412
Application Number = 19737
Date of Application = 22,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = METROGEL
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.75%
----
Product id = 5470
Application Number = 20208
Date of Application = 17,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = METROGEL-VAGINAL
Route/format = VAGINAL / GEL
Application Type = N
Applicant Name = MEDICIS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.75%
----
Product id = 1953
Application Number = 20334
Date of Application = 3,, May, 1995
RX/OTC/DISCN = RX
Tradename = FLAGYL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 375MG
----
Product id = 4305
Application Number = 20531
Date of Application = 20,, Sep, 1995
RX/OTC/DISCN = RX
Tradename = METROCREAM
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.75%
----
Product id = 4342
Application Number = 20743
Date of Application = 26,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = NORITATE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1%
----
Product id = 15962
Application Number = 20868
Date of Application = 26,, Nov, 1997
RX/OTC/DISCN = RX
Tradename = FLAGYL ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 750MG
----
Product id = 12234
Application Number = 20901
Date of Application = 24,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = METROLOTION
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.75%
----
Product id = 5413
Application Number = 21789
Date of Application = 30,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = METROGEL
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1%
----
Product id = 5473
Application Number = 21806
Date of Application = 20,, May, 2005
RX/OTC/DISCN = RX
Tradename = VANDAZOLE
Route/format = VAGINAL / GEL
Application Type = N
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = BX
Rld = No
Strength = 0.75%
----
Product id = 15191
Application Number = 50719
Date of Application = 15,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = HELIDAC
Route/format = ORAL / TABLET, CHEWABLE, TABLET, CAPSULE
Application Type = N
Applicant Name = PROMETHEUS LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 262.4MG,N/A,N/A;N/A,250MG,N/A;N/A,N/A,500MG
----
Product id = 3086
Application Number = 50786
Date of Application = 28,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = PYLERA
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = FOREST LABS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 140MG;125MG;125MG
----
Product id = 9300
Application Number = 70004
Date of Application = 8,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/100ML
----
Product id = 24891
Application Number = 70008
Date of Application = 11,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24892
Application Number = 70009
Date of Application = 11,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24875
Application Number = 70021
Date of Application = 2,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24893
Application Number = 70027
Date of Application = 6,, Nov, 1984
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 27838
Application Number = 70029
Date of Application = 19,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = SATRIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24886
Application Number = 70033
Date of Application = 6,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24898
Application Number = 70035
Date of Application = 20,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 24897
Application Number = 70039
Date of Application = 29,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VIVIMED LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24896
Application Number = 70040
Date of Application = 29,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VIVIMED LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 9301
Application Number = 70042
Date of Application = 20,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/100ML
----
Product id = 24903
Application Number = 70044
Date of Application = 8,, Feb, 1985
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 9298
Application Number = 70071
Date of Application = 3,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/100ML
----
Product id = 9302
Application Number = 70170
Date of Application = 1,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/100ML
----
Product id = 24876
Application Number = 70593
Date of Application = 27,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 27839
Application Number = 70731
Date of Application = 8,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = SATRIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24880
Application Number = 70772
Date of Application = 16,, Jul, 1986
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 24882
Application Number = 70773
Date of Application = 16,, Jul, 1986
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24869
Application Number = 74523
Date of Application = 24,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = METROMIDOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LABS AF
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24870
Application Number = 74523
Date of Application = 24,, Oct, 1996
RX/OTC/DISCN = DISCN
Tradename = METROMIDOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LABS AF
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 4306
Application Number = 76408
Date of Application = 28,, May, 2004
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 16241
Application Number = 76462
Date of Application = 25,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 2486
Application Number = 76505
Date of Application = 13,, Nov, 2003
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 24871
Application Number = 76519
Date of Application = 27,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 24872
Application Number = 76519
Date of Application = 27,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG
----
Product id = 2488
Application Number = 76522
Date of Application = 29,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 5414
Application Number = 77018
Date of Application = 6,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 12235
Application Number = 77197
Date of Application = 24,, May, 2006
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 5471
Application Number = 77264
Date of Application = 31,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = VAGINAL / GEL
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 5417
Application Number = 77547
Date of Application = 13,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 4307
Application Number = 77549
Date of Application = 19,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 5416
Application Number = 77819
Date of Application = 18,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 9304
Application Number = 78084
Date of Application = 31,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CLARIS LIFESCIENCES
ProductNo = 001
Tecode = AP
Rld = No
Strength = 500MG/100ML
----
Product id = 5415
Application Number = 78178
Date of Application = 19,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.75%
----
Product id = 2487
Application Number = 79065
Date of Application = 23,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 375MG
----
Product id = 24873
Application Number = 79067
Date of Application = 13,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 24874
Application Number = 79067
Date of Application = 13,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 16242
Application Number = 90222
Date of Application = 5,, May, 2010
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 5418
Application Number = 90903
Date of Application = 22,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1%
----
Product id = 24894
Application Number = 203458
Date of Application = 22,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 24895
Application Number = 203458
Date of Application = 22,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = METRONIDAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 5472
Application Number = 205223
Date of Application = 24,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = NUVESSA
Route/format = VAGINAL / GEL
Application Type = N
Applicant Name = ACTAVIS LABS UT INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1.3%
----