metoclopramide-hydrochloride

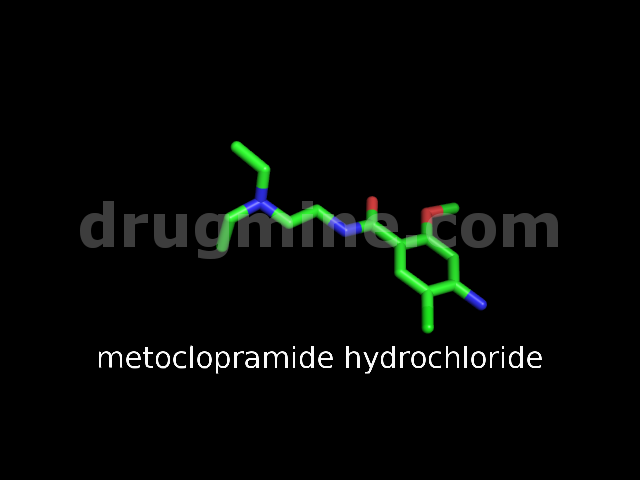
Name: METOCLOPRAMIDE HYDROCHLORIDE
ID :
MW: 300
Number of atoms: 20
Molecular_Formula: C14H22ClN3O2
Alogp: 1.775
Indication class : Anti-Emetic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
metoclopramide hydrochloride containing products summary
There are in total 75 different products containing the active ingredient metoclopramide hydrochloride. From the 75 drug products, 48 have been discontinued.Product id = 27396
Application Number = 17854
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = REGLAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ANI PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE
----
Product id = 27395
Application Number = 17854
Date of Application = 5,, May, 1987
RX/OTC/DISCN = RX
Tradename = REGLAN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ANI PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 10624
Application Number = 17862
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = REGLAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE CORP
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 5MG BASE/ML
----
Product id = 10625
Application Number = 17862
Date of Application = 28,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = REGLAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE CORP
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 14018
Application Number = 18821
Date of Application = 25,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = REGLAN
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = ROBINS AH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 17030
Application Number = 21793
Date of Application = 10,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = REGLAN ODT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17031
Application Number = 21793
Date of Application = 10,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = REGLAN ODT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = MEDA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 16931
Application Number = 22246
Date of Application = 4,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = METOZOLV ODT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = SALIX PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 5MG BASE
----
Product id = 16932
Application Number = 22246
Date of Application = 4,, Sep, 2009
RX/OTC/DISCN = DISCN
Tradename = METOZOLV ODT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = SALIX PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 24130
Application Number = 70106
Date of Application = 4,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = MAXOLON
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KING PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24798
Application Number = 70184
Date of Application = 29,, Jul, 1985
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9277
Application Number = 70293
Date of Application = 24,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LYPHOMED
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/2ML
----
Product id = 19887
Application Number = 70294
Date of Application = 29,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = CLOPRA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24799
Application Number = 70339
Date of Application = 29,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24788
Application Number = 70342
Date of Application = 25,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24803
Application Number = 70363
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24804
Application Number = 70453
Date of Application = 6,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9269
Application Number = 70505
Date of Application = 23,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9270
Application Number = 70506
Date of Application = 22,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 24805
Application Number = 70511
Date of Application = 22,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24789
Application Number = 70581
Date of Application = 17,, Oct, 1985
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24795
Application Number = 70598
Date of Application = 2,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9280
Application Number = 70622
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/2ML
----
Product id = 9279
Application Number = 70623
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 19888
Application Number = 70632
Date of Application = 28,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = CLOPRA-"YELLOW"
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24806
Application Number = 70645
Date of Application = 11,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24784
Application Number = 70660
Date of Application = 10,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 13937
Application Number = 70819
Date of Application = 10,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 9271
Application Number = 70847
Date of Application = 7,, Nov, 1988
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 24792
Application Number = 70850
Date of Application = 3,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9278
Application Number = 70892
Date of Application = 26,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = NORBROOK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/2ML
----
Product id = 24779
Application Number = 70906
Date of Application = 28,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24796
Application Number = 70926
Date of Application = 26,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 13932
Application Number = 70949
Date of Application = 6,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 24780
Application Number = 71213
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INTERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24807
Application Number = 71250
Date of Application = 3,, Feb, 1988
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9272
Application Number = 71291
Date of Application = 3,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 13938
Application Number = 71315
Date of Application = 30,, Jun, 1993
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 13930
Application Number = 71340
Date of Application = 18,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 13931
Application Number = 71402
Date of Application = 25,, Jun, 1993
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ANI PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 24785
Application Number = 71536
Date of Application = 28,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24783
Application Number = 71536
Date of Application = 16,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 13933
Application Number = 71665
Date of Application = 5,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PACO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 9273
Application Number = 71990
Date of Application = 18,, Jan, 1989
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 13935
Application Number = 72038
Date of Application = 5,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 9266
Application Number = 72155
Date of Application = 30,, Mar, 1992
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 24793
Application Number = 72215
Date of Application = 30,, Jan, 1990
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9267
Application Number = 72244
Date of Application = 30,, Mar, 1992
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9268
Application Number = 72247
Date of Application = 18,, May, 1992
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 19886
Application Number = 72384
Date of Application = 2,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = CLOPRA
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24790
Application Number = 72436
Date of Application = 22,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24778
Application Number = 72639
Date of Application = 9,, May, 1991
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CLONMEL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 13934
Application Number = 72744
Date of Application = 28,, May, 1991
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 24802
Application Number = 72750
Date of Application = 28,, Dec, 1995
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24797
Application Number = 72801
Date of Application = 15,, Jun, 1993
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 3953
Application Number = 72995
Date of Application = 30,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE INTENSOL
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 9274
Application Number = 73117
Date of Application = 17,, Jan, 1991
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9275
Application Number = 73118
Date of Application = 17,, Jan, 1991
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 9281
Application Number = 73135
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 13936
Application Number = 73680
Date of Application = 27,, Oct, 1992
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = SILARX
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 9276
Application Number = 74147
Date of Application = 2,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 24791
Application Number = 74478
Date of Application = 5,, Oct, 1995
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24794
Application Number = 74478
Date of Application = 5,, Oct, 1995
RX/OTC/DISCN = DISCN
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 13940
Application Number = 74703
Date of Application = 31,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = EQ 5MG BASE/5ML
----
Product id = 13939
Application Number = 75051
Date of Application = 26,, Jan, 2001
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = VISTAPHARM
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 5MG BASE/5ML
----
Product id = 24800
Application Number = 77878
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24801
Application Number = 77878
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24786
Application Number = 78374
Date of Application = 30,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24787
Application Number = 78374
Date of Application = 30,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 24781
Application Number = 78807
Date of Application = 12,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 24782
Application Number = 78807
Date of Application = 12,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9265
Application Number = 91392
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BD RX
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----
Product id = 16929
Application Number = 202191
Date of Application = 15,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 16930
Application Number = 202191
Date of Application = 15,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 9264
Application Number = 204756
Date of Application = 20,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = METOCLOPRAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EMCURE PHARMS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----