methylphenidate-hydrochloride
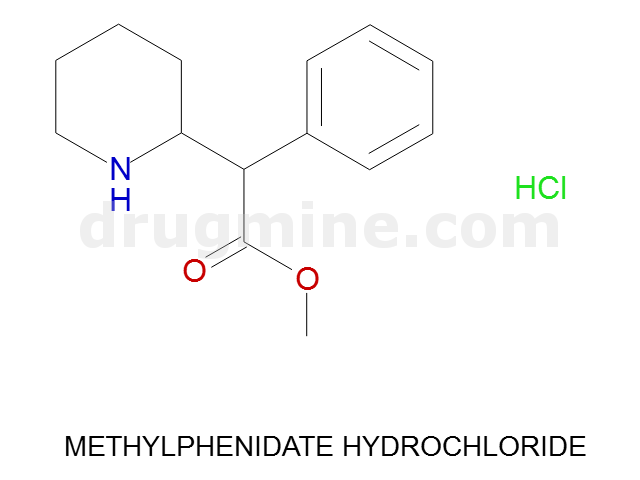
Name: METHYLPHENIDATE HYDROCHLORIDE
ID :
MW: 233
Number of atoms: 17
Molecular_Formula: C14H19NO2
Alogp: 2.18
Indication class : Stimulant (central)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
methylphenidate hydrochloride containing products summary
There are in total 83 different products containing the active ingredient methylphenidate hydrochloride. From the 83 drug products, 10 have been discontinued.Product id = 27686
Application Number = 10187
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RITALIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 27687
Application Number = 10187
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RITALIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 006
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 27688
Application Number = 10187
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = RITALIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 010
Tecode = AB
Rld = Yes
Strength = 20MG
----
Product id = 16579
Application Number = 18029
Date of Application = 30,, Mar, 1982
RX/OTC/DISCN = RX
Tradename = RITALIN-SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 15839
Application Number = 21121
Date of Application = 1,, Aug, 2000
RX/OTC/DISCN = RX
Tradename = CONCERTA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 18MG
----
Product id = 15841
Application Number = 21121
Date of Application = 1,, Aug, 2000
RX/OTC/DISCN = RX
Tradename = CONCERTA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 36MG
----
Product id = 15842
Application Number = 21121
Date of Application = 8,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = CONCERTA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 003
Tecode =
Rld = Yes
Strength = 54MG
----
Product id = 15840
Application Number = 21121
Date of Application = 1,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = CONCERTA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 27MG
----
Product id = 597
Application Number = 21259
Date of Application = 3,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = METADATE CD
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 20MG
----
Product id = 598
Application Number = 21259
Date of Application = 19,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = METADATE CD
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 30MG
----
Product id = 596
Application Number = 21259
Date of Application = 27,, May, 2003
RX/OTC/DISCN = RX
Tradename = METADATE CD
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 003
Tecode = AB2
Rld = No
Strength = 10MG
----
Product id = 599
Application Number = 21259
Date of Application = 19,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = METADATE CD
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 004
Tecode = AB2
Rld = No
Strength = 40MG
----
Product id = 600
Application Number = 21259
Date of Application = 19,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = METADATE CD
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 005
Tecode = AB2
Rld = No
Strength = 50MG
----
Product id = 601
Application Number = 21259
Date of Application = 19,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = METADATE CD
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = UCB INC
ProductNo = 006
Tecode = AB2
Rld = Yes
Strength = 60MG
----
Product id = 731
Application Number = 21284
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = RITALIN LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 20MG
----
Product id = 732
Application Number = 21284
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = RITALIN LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode = AB1
Rld = No
Strength = 30MG
----
Product id = 733
Application Number = 21284
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = RITALIN LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB1
Rld = No
Strength = 40MG
----
Product id = 730
Application Number = 21284
Date of Application = 10,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = RITALIN LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode = AB1
Rld = No
Strength = 10MG
----
Product id = 734
Application Number = 21284
Date of Application = 27,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = RITALIN LA
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = Yes
Strength = 60MG
----
Product id = 13924
Application Number = 21419
Date of Application = 19,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = METHYLIN
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 5MG/5ML
----
Product id = 13925
Application Number = 21419
Date of Application = 19,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = METHYLIN
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = MALLINCKRODT
ProductNo = 002
Tecode = AA
Rld = Yes
Strength = 10MG/5ML
----
Product id = 15305
Application Number = 21475
Date of Application = 15,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = METHYLIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 15306
Application Number = 21475
Date of Application = 15,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = METHYLIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MALLINCKRODT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 15307
Application Number = 21475
Date of Application = 15,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = METHYLIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MALLINCKRODT
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 24722
Application Number = 40220
Date of Application = 29,, Aug, 1997
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24723
Application Number = 40220
Date of Application = 29,, Aug, 1997
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24724
Application Number = 40220
Date of Application = 29,, Aug, 1997
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24728
Application Number = 40300
Date of Application = 27,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24729
Application Number = 40300
Date of Application = 27,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24730
Application Number = 40300
Date of Application = 27,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 16161
Application Number = 40306
Date of Application = 20,, Oct, 1999
RX/OTC/DISCN = DISCN
Tradename = METADATE ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24719
Application Number = 40321
Date of Application = 5,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 24720
Application Number = 40321
Date of Application = 5,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24721
Application Number = 40321
Date of Application = 5,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG
----
Product id = 24716
Application Number = 40404
Date of Application = 29,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 24717
Application Number = 40404
Date of Application = 29,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24718
Application Number = 40404
Date of Application = 29,, Mar, 2001
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG
----
Product id = 16216
Application Number = 40410
Date of Application = 9,, Feb, 2001
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 16208
Application Number = 75450
Date of Application = 21,, Dec, 2001
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 16205
Application Number = 75629
Date of Application = 9,, May, 2000
RX/OTC/DISCN = RX
Tradename = METHYLIN ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MALLINCKRODT INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 16206
Application Number = 75629
Date of Application = 9,, May, 2000
RX/OTC/DISCN = RX
Tradename = METHYLIN ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MALLINCKRODT INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 16207
Application Number = 76032
Date of Application = 9,, May, 2001
RX/OTC/DISCN = DISCN
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 606
Application Number = 77707
Date of Application = 19,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 10MG
----
Product id = 607
Application Number = 77707
Date of Application = 19,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 20MG
----
Product id = 608
Application Number = 77707
Date of Application = 19,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB2
Rld = No
Strength = 30MG
----
Product id = 612
Application Number = 78458
Date of Application = 1,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 20MG
----
Product id = 613
Application Number = 78458
Date of Application = 1,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode = AB1
Rld = No
Strength = 30MG
----
Product id = 614
Application Number = 78458
Date of Application = 1,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode = AB1
Rld = No
Strength = 40MG
----
Product id = 609
Application Number = 78873
Date of Application = 19,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 40MG
----
Product id = 610
Application Number = 78873
Date of Application = 19,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 50MG
----
Product id = 611
Application Number = 78873
Date of Application = 19,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB2
Rld = No
Strength = 60MG
----
Product id = 603
Application Number = 79031
Date of Application = 13,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 20MG
----
Product id = 604
Application Number = 79031
Date of Application = 13,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 002
Tecode = AB1
Rld = No
Strength = 30MG
----
Product id = 605
Application Number = 79031
Date of Application = 13,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 003
Tecode = AB1
Rld = No
Strength = 40MG
----
Product id = 602
Application Number = 79031
Date of Application = 15,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR LABS INC
ProductNo = 004
Tecode = AB1
Rld = No
Strength = 10MG
----
Product id = 24735
Application Number = 85799
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24736
Application Number = 86428
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24734
Application Number = 86429
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 16162
Application Number = 89601
Date of Application = 1,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = METADATE ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 20MG
----
Product id = 24731
Application Number = 90710
Date of Application = 15,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24732
Application Number = 90710
Date of Application = 15,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24733
Application Number = 90710
Date of Application = 15,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24725
Application Number = 91159
Date of Application = 12,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24726
Application Number = 91159
Date of Application = 12,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24727
Application Number = 91159
Date of Application = 12,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 13928
Application Number = 91601
Date of Application = 23,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 5MG/5ML
----
Product id = 13929
Application Number = 91601
Date of Application = 23,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TRIS PHARMA INC
ProductNo = 002
Tecode = AA
Rld = No
Strength = 10MG/5ML
----
Product id = 16209
Application Number = 91695
Date of Application = 9,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 001
Tecode = BX
Rld = No
Strength = 18MG
----
Product id = 16210
Application Number = 91695
Date of Application = 9,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 002
Tecode = BX
Rld = No
Strength = 27MG
----
Product id = 16211
Application Number = 91695
Date of Application = 23,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 003
Tecode = BX
Rld = No
Strength = 36MG
----
Product id = 16212
Application Number = 91695
Date of Application = 23,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 004
Tecode = BX
Rld = No
Strength = 54MG
----
Product id = 13926
Application Number = 201466
Date of Application = 12,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 5MG/5ML
----
Product id = 13927
Application Number = 201466
Date of Application = 12,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AA
Rld = No
Strength = 10MG/5ML
----
Product id = 4979
Application Number = 202100
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = QUILLIVANT XR
Route/format = ORAL / FOR SUSPENSION, EXTENDED RELEASE
Application Type = N
Applicant Name = NEXTWAVE PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MG/ML
----
Product id = 16213
Application Number = 202608
Date of Application = 28,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MALLINCKRODT INC
ProductNo = 001
Tecode = BX
Rld = No
Strength = 27MG
----
Product id = 16214
Application Number = 202608
Date of Application = 28,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MALLINCKRODT INC
ProductNo = 002
Tecode = BX
Rld = No
Strength = 36MG
----
Product id = 16215
Application Number = 202608
Date of Application = 28,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MALLINCKRODT INC
ProductNo = 003
Tecode = BX
Rld = No
Strength = 54MG
----
Product id = 24737
Application Number = 202892
Date of Application = 23,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24738
Application Number = 202892
Date of Application = 23,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24739
Application Number = 202892
Date of Application = 23,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 15308
Application Number = 204115
Date of Application = 25,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 15309
Application Number = 204115
Date of Application = 25,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 15310
Application Number = 204115
Date of Application = 25,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = METHYLPHENIDATE HYDROCHLORIDE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = NOVEL LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----