metformin-hydrochloride
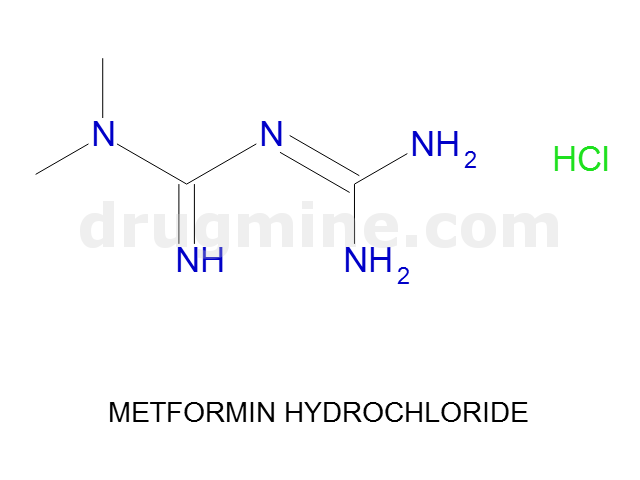

Name: METFORMIN HYDROCHLORIDE
ID :
MW: 129
Number of atoms: 9
Molecular_Formula: C4H11N5
Alogp: -1.388
Indication class : Antidiabetic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
metformin hydrochloride containing products summary
There are in total 239 different products containing the active ingredient metformin hydrochloride. From the 239 drug products, 36 have been discontinued.Product id = 21884
Application Number = 20357
Date of Application = 3,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = GLUCOPHAGE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 21887
Application Number = 20357
Date of Application = 3,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = GLUCOPHAGE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 21885
Application Number = 20357
Date of Application = 5,, Nov, 1998
RX/OTC/DISCN = DISCN
Tradename = GLUCOPHAGE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 625MG
----
Product id = 21886
Application Number = 20357
Date of Application = 5,, Nov, 1998
RX/OTC/DISCN = DISCN
Tradename = GLUCOPHAGE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode =
Rld = No
Strength = 750MG
----
Product id = 21883
Application Number = 20357
Date of Application = 5,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = GLUCOPHAGE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 1GM
----
Product id = 21891
Application Number = 21178
Date of Application = 31,, Jul, 2000
RX/OTC/DISCN = RX
Tradename = GLUCOVANCE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1.25MG;250MG
----
Product id = 21892
Application Number = 21178
Date of Application = 31,, Jul, 2000
RX/OTC/DISCN = RX
Tradename = GLUCOVANCE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 2.5MG;500MG
----
Product id = 21893
Application Number = 21178
Date of Application = 31,, Jul, 2000
RX/OTC/DISCN = RX
Tradename = GLUCOVANCE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 15971
Application Number = 21202
Date of Application = 13,, Oct, 2000
RX/OTC/DISCN = RX
Tradename = GLUCOPHAGE XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 15972
Application Number = 21202
Date of Application = 11,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = GLUCOPHAGE XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 750MG
----
Product id = 18224
Application Number = 21410
Date of Application = 10,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;EQ 1MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18225
Application Number = 21410
Date of Application = 10,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG;EQ 2MG BASE
----
Product id = 18226
Application Number = 21410
Date of Application = 10,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 500MG;EQ 4MG BASE
----
Product id = 18222
Application Number = 21410
Date of Application = 25,, Aug, 2003
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 004
Tecode = AB
Rld = No
Strength = 1GM;EQ 2MG BASE
----
Product id = 18223
Application Number = 21410
Date of Application = 25,, Aug, 2003
RX/OTC/DISCN = RX
Tradename = AVANDAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SB PHARMCO
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 1GM;EQ 4MG BASE
----
Product id = 24373
Application Number = 21460
Date of Application = 21,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = METAGLIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;250MG
----
Product id = 24374
Application Number = 21460
Date of Application = 21,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = METAGLIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG;500MG
----
Product id = 24375
Application Number = 21460
Date of Application = 21,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = METAGLIP
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG;500MG
----
Product id = 15965
Application Number = 21574
Date of Application = 27,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = FORTAMET
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANDRX LABS LLC
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 500MG
----
Product id = 15964
Application Number = 21574
Date of Application = 27,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = FORTAMET
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANDRX LABS LLC
ProductNo = 002
Tecode = AB2
Rld = Yes
Strength = 1GM
----
Product id = 14020
Application Number = 21591
Date of Application = 11,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = RIOMET
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = Yes
Strength = 500MG/5ML
----
Product id = 15977
Application Number = 21748
Date of Application = 3,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = GLUMETZA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 15976
Application Number = 21748
Date of Application = 3,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = GLUMETZA
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SANTARUS INC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1GM
----
Product id = 17329
Application Number = 21842
Date of Application = 29,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 17330
Application Number = 21842
Date of Application = 29,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 850MG;EQ 15MG BASE
----
Product id = 15628
Application Number = 22024
Date of Application = 12,, May, 2009
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM;EQ 15MG BASE
----
Product id = 15629
Application Number = 22024
Date of Application = 12,, May, 2009
RX/OTC/DISCN = RX
Tradename = ACTOPLUS MET XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1GM;EQ 30MG BASE
----
Product id = 23120
Application Number = 22044
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = JANUMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;EQ 50MG BASE
----
Product id = 23119
Application Number = 22044
Date of Application = 30,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = JANUMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1GM;EQ 50MG BASE
----
Product id = 26425
Application Number = 22386
Date of Application = 23,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = PRANDIMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVO NORDISK INC
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;1MG
----
Product id = 26426
Application Number = 22386
Date of Application = 23,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = PRANDIMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVO NORDISK INC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 500MG;2MG
----
Product id = 24482
Application Number = 75961
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS FLORIDA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24483
Application Number = 75961
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS FLORIDA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24481
Application Number = 75961
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS FLORIDA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24459
Application Number = 75965
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24461
Application Number = 75965
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24457
Application Number = 75965
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24467
Application Number = 75967
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24468
Application Number = 75967
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24466
Application Number = 75967
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24452
Application Number = 75969
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24453
Application Number = 75969
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24451
Application Number = 75969
Date of Application = 29,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24412
Application Number = 75971
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24413
Application Number = 75971
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode =
Rld = No
Strength = 850MG
----
Product id = 24411
Application Number = 75971
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM
----
Product id = 24415
Application Number = 75972
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CHARTWELL LIFE SCI
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24418
Application Number = 75972
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CHARTWELL LIFE SCI
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24414
Application Number = 75972
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CHARTWELL LIFE SCI
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24417
Application Number = 75972
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CHARTWELL LIFE SCI
ProductNo = 004
Tecode =
Rld = No
Strength = 750MG
----
Product id = 24416
Application Number = 75972
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CHARTWELL LIFE SCI
ProductNo = 005
Tecode =
Rld = No
Strength = 625MG
----
Product id = 24447
Application Number = 75973
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24449
Application Number = 75973
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24445
Application Number = 75973
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24435
Application Number = 75975
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24438
Application Number = 75975
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 850MG
----
Product id = 24434
Application Number = 75975
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM
----
Product id = 24436
Application Number = 75975
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 625MG
----
Product id = 24437
Application Number = 75975
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = 750MG
----
Product id = 24448
Application Number = 75976
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24450
Application Number = 75976
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24446
Application Number = 75976
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24471
Application Number = 75978
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24473
Application Number = 75978
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24469
Application Number = 75978
Date of Application = 5,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24479
Application Number = 75979
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24480
Application Number = 75979
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 850MG
----
Product id = 24478
Application Number = 75979
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM
----
Product id = 24401
Application Number = 75984
Date of Application = 23,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24403
Application Number = 75984
Date of Application = 23,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24399
Application Number = 75984
Date of Application = 23,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24460
Application Number = 75985
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24462
Application Number = 75985
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24458
Application Number = 75985
Date of Application = 25,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24406
Application Number = 76033
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ATLAS PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24407
Application Number = 76033
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ATLAS PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24405
Application Number = 76033
Date of Application = 24,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ATLAS PHARMS LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24443
Application Number = 76038
Date of Application = 21,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24444
Application Number = 76038
Date of Application = 21,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24442
Application Number = 76038
Date of Application = 21,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 16165
Application Number = 76172
Date of Application = 16,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16194
Application Number = 76223
Date of Application = 14,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16177
Application Number = 76249
Date of Application = 30,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16198
Application Number = 76269
Date of Application = 18,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 24472
Application Number = 76328
Date of Application = 16,, Dec, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24474
Application Number = 76328
Date of Application = 16,, Dec, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 850MG
----
Product id = 24470
Application Number = 76328
Date of Application = 16,, Dec, 2002
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM
----
Product id = 21949
Application Number = 76345
Date of Application = 18,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1.25MG;250MG
----
Product id = 21950
Application Number = 76345
Date of Application = 18,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21951
Application Number = 76345
Date of Application = 18,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 16192
Application Number = 76413
Date of Application = 18,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16163
Application Number = 76450
Date of Application = 1,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16173
Application Number = 76496
Date of Application = 25,, Nov, 2005
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16180
Application Number = 76545
Date of Application = 1,, Dec, 2003
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16186
Application Number = 76650
Date of Application = 13,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16169
Application Number = 76706
Date of Application = 14,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16170
Application Number = 76706
Date of Application = 29,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 21937
Application Number = 76716
Date of Application = 28,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1.25MG;250MG
----
Product id = 21938
Application Number = 76716
Date of Application = 28,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21939
Application Number = 76716
Date of Application = 28,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21943
Application Number = 76731
Date of Application = 19,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1.25MG;250MG
----
Product id = 21944
Application Number = 76731
Date of Application = 19,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21945
Application Number = 76731
Date of Application = 19,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 16190
Application Number = 76756
Date of Application = 26,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = NOSTRUM PHARMS LLC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16191
Application Number = 76756
Date of Application = 12,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = NOSTRUM PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 16202
Application Number = 76818
Date of Application = 14,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 21952
Application Number = 76821
Date of Application = 27,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 1.25MG;250MG
----
Product id = 21953
Application Number = 76821
Date of Application = 27,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21954
Application Number = 76821
Date of Application = 27,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG;500MG
----
Product id = 16174
Application Number = 76863
Date of Application = 14,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 16199
Application Number = 76864
Date of Application = 12,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 16166
Application Number = 76869
Date of Application = 12,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 16195
Application Number = 76873
Date of Application = 14,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16164
Application Number = 76878
Date of Application = 13,, Apr, 2005
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 16178
Application Number = 76985
Date of Application = 13,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 16203
Application Number = 77060
Date of Application = 20,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 24488
Application Number = 77064
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24489
Application Number = 77064
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24487
Application Number = 77064
Date of Application = 18,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 16204
Application Number = 77078
Date of Application = 21,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 24409
Application Number = 77095
Date of Application = 14,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24410
Application Number = 77095
Date of Application = 14,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24408
Application Number = 77095
Date of Application = 14,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 16187
Application Number = 77113
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 16185
Application Number = 77124
Date of Application = 21,, Dec, 2005
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16193
Application Number = 77211
Date of Application = 29,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 21876
Application Number = 77270
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21877
Application Number = 77270
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21878
Application Number = 77270
Date of Application = 28,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 5MG;500MG
----
Product id = 16196
Application Number = 77336
Date of Application = 9,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS (IN)
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16197
Application Number = 77336
Date of Application = 9,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS (IN)
ProductNo = 002
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 27803
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 2MG BASE
----
Product id = 27804
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 500MG;EQ 4MG BASE
----
Product id = 27802
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM;EQ 2MG BASE
----
Product id = 27801
Application Number = 77337
Date of Application = 7,, May, 2014
RX/OTC/DISCN = RX
Tradename = ROSIGLITAZONE MALEATE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 1GM;EQ 4MG BASE
----
Product id = 21864
Application Number = 77507
Date of Application = 27,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21865
Application Number = 77507
Date of Application = 27,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21866
Application Number = 77507
Date of Application = 27,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21873
Application Number = 77620
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21874
Application Number = 77620
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21875
Application Number = 77620
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 24476
Application Number = 77711
Date of Application = 24,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24477
Application Number = 77711
Date of Application = 24,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24475
Application Number = 77711
Date of Application = 24,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24420
Application Number = 77787
Date of Application = 23,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24421
Application Number = 77787
Date of Application = 23,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24419
Application Number = 77787
Date of Application = 23,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24455
Application Number = 77853
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROVIDENT PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24456
Application Number = 77853
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROVIDENT PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24454
Application Number = 77853
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROVIDENT PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 21940
Application Number = 77870
Date of Application = 14,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1.25MG;250MG
----
Product id = 21941
Application Number = 77870
Date of Application = 14,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21942
Application Number = 77870
Date of Application = 14,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 24397
Application Number = 77880
Date of Application = 5,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24398
Application Number = 77880
Date of Application = 5,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24396
Application Number = 77880
Date of Application = 5,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 21870
Application Number = 78083
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21871
Application Number = 78083
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21872
Application Number = 78083
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 24423
Application Number = 78170
Date of Application = 23,, May, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24424
Application Number = 78170
Date of Application = 23,, May, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24422
Application Number = 78170
Date of Application = 23,, May, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 16175
Application Number = 78321
Date of Application = 17,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = CSPC OUYI PHARM CO
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16176
Application Number = 78321
Date of Application = 17,, Apr, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = CSPC OUYI PHARM CO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 24432
Application Number = 78422
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 24433
Application Number = 78422
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 850MG
----
Product id = 24431
Application Number = 78422
Date of Application = 6,, Aug, 2007
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM
----
Product id = 16167
Application Number = 78596
Date of Application = 3,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16168
Application Number = 78596
Date of Application = 3,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 21867
Application Number = 78728
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21868
Application Number = 78728
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21869
Application Number = 78728
Date of Application = 23,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21879
Application Number = 78905
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;250MG
----
Product id = 21880
Application Number = 78905
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21881
Application Number = 78905
Date of Application = 31,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = GLIPIZIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 21946
Application Number = 79009
Date of Application = 3,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1.25MG;250MG
----
Product id = 21947
Application Number = 79009
Date of Application = 3,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG;500MG
----
Product id = 21948
Application Number = 79009
Date of Application = 3,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = GLYBURIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;500MG
----
Product id = 16171
Application Number = 79118
Date of Application = 20,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 16172
Application Number = 79118
Date of Application = 20,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 750MG
----
Product id = 24429
Application Number = 79148
Date of Application = 25,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INDICUS PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24430
Application Number = 79148
Date of Application = 25,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INDICUS PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24428
Application Number = 79148
Date of Application = 25,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INDICUS PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 16201
Application Number = 79226
Date of Application = 18,, Feb, 2010
RX/OTC/DISCN = DISCN
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 16200
Application Number = 90014
Date of Application = 30,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARM
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 26300
Application Number = 90406
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26301
Application Number = 90406
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 24426
Application Number = 90564
Date of Application = 22,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GRANULES INDIA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24427
Application Number = 90564
Date of Application = 22,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GRANULES INDIA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24425
Application Number = 90564
Date of Application = 22,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GRANULES INDIA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24402
Application Number = 90666
Date of Application = 7,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24404
Application Number = 90666
Date of Application = 7,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24400
Application Number = 90666
Date of Application = 7,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 16183
Application Number = 90692
Date of Application = 29,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 500MG
----
Product id = 16181
Application Number = 90692
Date of Application = 29,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 1GM
----
Product id = 24440
Application Number = 90888
Date of Application = 12,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24441
Application Number = 90888
Date of Application = 12,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24439
Application Number = 90888
Date of Application = 12,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MARKSANS PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 26304
Application Number = 91155
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26305
Application Number = 91155
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 24394
Application Number = 91184
Date of Application = 1,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALKEM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24395
Application Number = 91184
Date of Application = 1,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALKEM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24393
Application Number = 91184
Date of Application = 1,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALKEM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 26302
Application Number = 91273
Date of Application = 16,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26303
Application Number = 91273
Date of Application = 16,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 16184
Application Number = 91664
Date of Application = 19,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB3
Rld = No
Strength = 500MG
----
Product id = 16182
Application Number = 91664
Date of Application = 19,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB3
Rld = No
Strength = 1GM
----
Product id = 16058
Application Number = 200678
Date of Application = 5,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = KOMBIGLYZE XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ASTRAZENECA AB
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;EQ 5MG BASE
----
Product id = 16057
Application Number = 200678
Date of Application = 5,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = KOMBIGLYZE XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ASTRAZENECA AB
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1GM;EQ 5MG BASE
----
Product id = 16056
Application Number = 200678
Date of Application = 5,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = KOMBIGLYZE XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ASTRAZENECA AB
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM;EQ 2.5MG BASE
----
Product id = 16189
Application Number = 200690
Date of Application = 1,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 500MG
----
Product id = 16188
Application Number = 200690
Date of Application = 1,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB2
Rld = No
Strength = 1GM
----
Product id = 26298
Application Number = 200823
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26299
Application Number = 200823
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 23127
Application Number = 201281
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = JENTADUETO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;500MG
----
Product id = 23128
Application Number = 201281
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = JENTADUETO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG;850MG
----
Product id = 23126
Application Number = 201281
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = JENTADUETO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode =
Rld = Yes
Strength = 2.5MG;1GM
----
Product id = 16179
Application Number = 201991
Date of Application = 18,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = INVENTIA HLTHCARE
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 500MG
----
Product id = 26306
Application Number = 202001
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG;EQ 15MG BASE
----
Product id = 26307
Application Number = 202001
Date of Application = 13,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG;EQ 15MG BASE
----
Product id = 16034
Application Number = 202270
Date of Application = 2,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = JANUMET XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG;EQ 50MG BASE
----
Product id = 16032
Application Number = 202270
Date of Application = 2,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = JANUMET XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode =
Rld = No
Strength = 1GM;EQ 50MG BASE
----
Product id = 16033
Application Number = 202270
Date of Application = 2,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = JANUMET XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 003
Tecode =
Rld = Yes
Strength = 1GM;EQ 100MG BASE
----
Product id = 23142
Application Number = 203414
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = KAZANO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12.5MG BASE;500MG
----
Product id = 23141
Application Number = 203414
Date of Application = 25,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = KAZANO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TAKEDA PHARMS USA
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 12.5MG BASE;1GM
----
Product id = 24485
Application Number = 203686
Date of Application = 28,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24486
Application Number = 203686
Date of Application = 28,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24484
Application Number = 203686
Date of Application = 28,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 24463
Application Number = 203769
Date of Application = 11,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCIEGEN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 500MG
----
Product id = 24464
Application Number = 203769
Date of Application = 11,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCIEGEN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 850MG
----
Product id = 24465
Application Number = 203769
Date of Application = 11,, Sep, 2013
RX/OTC/DISCN = RX
Tradename = METFORMIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCIEGEN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1GM
----
Product id = 22944
Application Number = 204353
Date of Application = 8,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = INVOKAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 22945
Application Number = 204353
Date of Application = 8,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = INVOKAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG;1GM
----
Product id = 22946
Application Number = 204353
Date of Application = 8,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = INVOKAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG;500MG
----
Product id = 22947
Application Number = 204353
Date of Application = 8,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = INVOKAMET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 004
Tecode =
Rld = Yes
Strength = 150MG;1GM
----
Product id = 16790
Application Number = 205649
Date of Application = 29,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = XIGDUO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ASTRAZENECA AB
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;500MG
----
Product id = 16789
Application Number = 205649
Date of Application = 29,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = XIGDUO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ASTRAZENECA AB
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;1GM
----
Product id = 16792
Application Number = 205649
Date of Application = 29,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = XIGDUO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ASTRAZENECA AB
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;500MG
----
Product id = 16791
Application Number = 205649
Date of Application = 29,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = XIGDUO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ASTRAZENECA AB
ProductNo = 004
Tecode =
Rld = Yes
Strength = 10MG;1GM
----