lovastatin
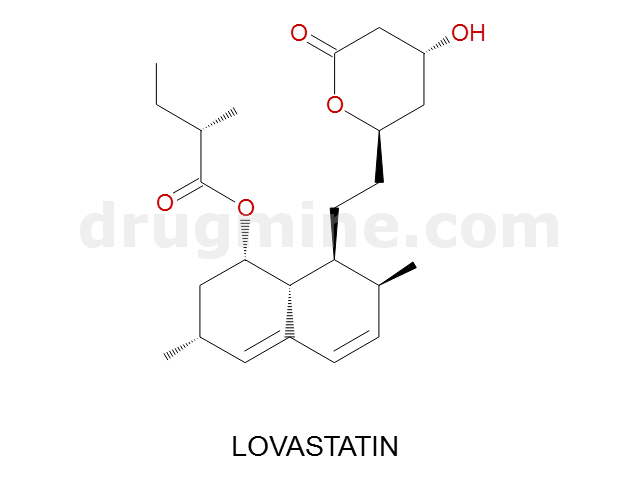


Name: LOVASTATIN
ID :
MW: 405
Number of atoms: 29
Molecular_Formula: C24H36O5
Alogp: 4.218
Indication class : Inhibitor (HMG-CoA reductase); Antihyperlipidemic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
lovastatin containing products summary
There are in total 41 different products containing the active ingredient lovastatin. From the 41 drug products, 4 have been discontinued.Product id = 24904
Application Number = 19643
Date of Application = 28,, Mar, 1991
RX/OTC/DISCN = DISCN
Tradename = MEVACOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 24905
Application Number = 19643
Date of Application = 31,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = MEVACOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG
----
Product id = 24906
Application Number = 19643
Date of Application = 14,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = MEVACOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 004
Tecode =
Rld = No
Strength = 40MG
----
Product id = 15634
Application Number = 21249
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = ADVICOR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = Yes
Strength = 20MG;500MG
----
Product id = 15635
Application Number = 21249
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = ADVICOR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 20MG;750MG
----
Product id = 15633
Application Number = 21249
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = ADVICOR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 003
Tecode =
Rld = Yes
Strength = 20MG;1GM
----
Product id = 15636
Application Number = 21249
Date of Application = 27,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = ADVICOR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 004
Tecode =
Rld = Yes
Strength = 40MG;1GM
----
Product id = 15708
Application Number = 21316
Date of Application = 26,, Jun, 2002
RX/OTC/DISCN = DISCN
Tradename = ALTOPREV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANDRX LABS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 15709
Application Number = 21316
Date of Application = 26,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = ALTOPREV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANDRX LABS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 15710
Application Number = 21316
Date of Application = 26,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = ALTOPREV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANDRX LABS LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 40MG
----
Product id = 15711
Application Number = 21316
Date of Application = 26,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = ALTOPREV
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANDRX LABS LLC
ProductNo = 004
Tecode =
Rld = Yes
Strength = 60MG
----
Product id = 24070
Application Number = 75300
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24072
Application Number = 75300
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24074
Application Number = 75300
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24064
Application Number = 75451
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24066
Application Number = 75451
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24068
Application Number = 75451
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24078
Application Number = 75551
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24077
Application Number = 75551
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24076
Application Number = 75551
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24071
Application Number = 75636
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24073
Application Number = 75636
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24075
Application Number = 75636
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24049
Application Number = 75828
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24050
Application Number = 75828
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24051
Application Number = 75828
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24065
Application Number = 75935
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24067
Application Number = 75935
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24069
Application Number = 75935
Date of Application = 17,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24055
Application Number = 75991
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24056
Application Number = 75991
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24057
Application Number = 75991
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 40MG
----
Product id = 24061
Application Number = 77520
Date of Application = 14,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24062
Application Number = 77520
Date of Application = 14,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24063
Application Number = 77520
Date of Application = 14,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24052
Application Number = 77748
Date of Application = 28,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24053
Application Number = 77748
Date of Application = 28,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24054
Application Number = 77748
Date of Application = 28,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24058
Application Number = 78296
Date of Application = 14,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24059
Application Number = 78296
Date of Application = 1,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24060
Application Number = 78296
Date of Application = 1,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = LOVASTATIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----