loratadine
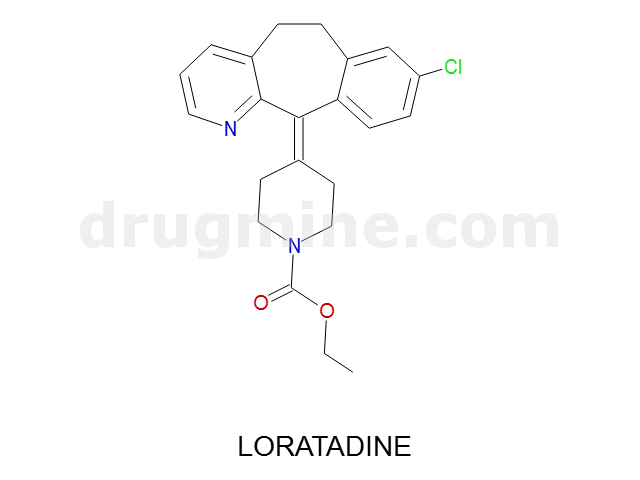
Name: LORATADINE
ID :
MW: 383
Number of atoms: 27
Molecular_Formula: C22H23ClN2O2
Alogp: 5
Indication class : Antihistaminic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
loratadine containing products summary
There are in total 37 different products containing the active ingredient loratadine. From the 37 drug products, 5 have been discontinued.Product id = 19753
Application Number = 19658
Date of Application = 27,, Nov, 2002
RX/OTC/DISCN = OTC
Tradename = CLARITIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 10MG
----
Product id = 19754
Application Number = 19658
Date of Application = 19,, Nov, 2003
RX/OTC/DISCN = OTC
Tradename = CLARITIN HIVES RELIEF
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 003
Tecode =
Rld = Yes
Strength = 10MG
----
Product id = 15831
Application Number = 19670
Date of Application = 27,, Nov, 2002
RX/OTC/DISCN = OTC
Tradename = CLARITIN-D
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 002
Tecode =
Rld = Yes
Strength = 5MG;120MG
----
Product id = 15832
Application Number = 20470
Date of Application = 27,, Nov, 2002
RX/OTC/DISCN = OTC
Tradename = CLARITIN-D 24 HOUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 002
Tecode =
Rld = Yes
Strength = 10MG;240MG
----
Product id = 14963
Application Number = 20641
Date of Application = 27,, Nov, 2002
RX/OTC/DISCN = OTC
Tradename = CLARITIN
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1MG/ML
----
Product id = 14964
Application Number = 20641
Date of Application = 19,, Nov, 2003
RX/OTC/DISCN = DISCN
Tradename = CLARITIN HIVES RELIEF
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16871
Application Number = 20704
Date of Application = 27,, Nov, 2002
RX/OTC/DISCN = OTC
Tradename = CLARITIN REDITABS
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 002
Tecode =
Rld = Yes
Strength = 10MG
----
Product id = 16870
Application Number = 20704
Date of Application = 19,, Nov, 2003
RX/OTC/DISCN = OTC
Tradename = CLARITIN HIVES RELIEF REDITAB
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = BAYER HEALTHCARE LLC
ProductNo = 003
Tecode =
Rld = Yes
Strength = 10MG
----
Product id = 16838
Application Number = 21375
Date of Application = 19,, Dec, 2002
RX/OTC/DISCN = OTC
Tradename = ALAVERT
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23873
Application Number = 21512
Date of Application = 24,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 14730
Application Number = 21734
Date of Application = 4,, Oct, 2005
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1MG/ML
----
Product id = 15241
Application Number = 21891
Date of Application = 23,, Aug, 2006
RX/OTC/DISCN = OTC
Tradename = CHILDREN'S CLARITIN
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MG
----
Product id = 1417
Application Number = 21952
Date of Application = 16,, Jun, 2008
RX/OTC/DISCN = OTC
Tradename = CLARITIN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 001
Tecode =
Rld = Yes
Strength = 10MG
----
Product id = 16872
Application Number = 21993
Date of Application = 12,, Dec, 2006
RX/OTC/DISCN = OTC
Tradename = CLARITIN REDITABS
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 001
Tecode =
Rld = Yes
Strength = 5MG
----
Product id = 23875
Application Number = 75209
Date of Application = 21,, Jan, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 15027
Application Number = 75505
Date of Application = 7,, Nov, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 15022
Application Number = 75565
Date of Application = 5,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = LORATADINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 16155
Application Number = 75706
Date of Application = 21,, Feb, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE AND PSEUDOEPHEDRINE SULFATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;240MG
----
Product id = 15023
Application Number = 75728
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 23869
Application Number = 75790
Date of Application = 7,, Nov, 2008
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 15028
Application Number = 75815
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 16925
Application Number = 75822
Date of Application = 10,, Feb, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 16157
Application Number = 75989
Date of Application = 4,, Mar, 2004
RX/OTC/DISCN = OTC
Tradename = LORATADINE AND PSEUDOEPHEDRINE SULFATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;240MG
----
Product id = 16923
Application Number = 75990
Date of Application = 3,, Nov, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 16924
Application Number = 76011
Date of Application = 29,, Sep, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 16156
Application Number = 76050
Date of Application = 30,, Jan, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE AND PSEUDOEPHEDRINE SULFATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;120MG
----
Product id = 23874
Application Number = 76134
Date of Application = 18,, Aug, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23870
Application Number = 76154
Date of Application = 20,, Aug, 2003
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 16154
Application Number = 76208
Date of Application = 28,, Jan, 2004
RX/OTC/DISCN = DISCN
Tradename = LORATADINE AND PSEUDOEPHEDRINE SULFATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;120MG
----
Product id = 23872
Application Number = 76301
Date of Application = 25,, Jun, 2004
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23868
Application Number = 76471
Date of Application = 14,, Feb, 2006
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 15024
Application Number = 76529
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = DISCN
Tradename = LORATADINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 16158
Application Number = 76557
Date of Application = 22,, Sep, 2004
RX/OTC/DISCN = OTC
Tradename = LORATADINE AND PSEUDOEPHEDRINE SULFATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;240MG
----
Product id = 15026
Application Number = 76805
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 16926
Application Number = 77153
Date of Application = 11,, Apr, 2007
RX/OTC/DISCN = OTC
Tradename = LORATADINE REDIDOSE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 15025
Application Number = 77421
Date of Application = 29,, Jun, 2006
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = SILARX
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 23871
Application Number = 78447
Date of Application = 12,, Aug, 2011
RX/OTC/DISCN = OTC
Tradename = LORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----