lisinopril
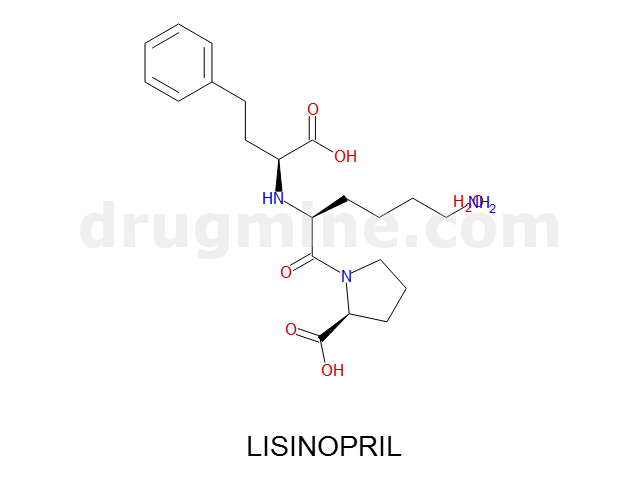
Name: LISINOPRIL
ID :
MW: 405
Number of atoms: 29
Molecular_Formula: C21H31N3O5
Alogp: -3.673
Indication class : Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
lisinopril containing products summary
There are in total 155 different products containing the active ingredient lisinopril. From the 155 drug products, 26 have been discontinued.Product id = 26689
Application Number = 19558
Date of Application = 29,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = PRINIVIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 26690
Application Number = 19558
Date of Application = 29,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = PRINIVIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 26691
Application Number = 19558
Date of Application = 29,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = PRINIVIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 26692
Application Number = 19558
Date of Application = 25,, Oct, 1988
RX/OTC/DISCN = RX
Tradename = PRINIVIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 26688
Application Number = 19558
Date of Application = 28,, Jan, 1994
RX/OTC/DISCN = DISCN
Tradename = PRINIVIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 006
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 29774
Application Number = 19777
Date of Application = 19,, May, 1988
RX/OTC/DISCN = RX
Tradename = ZESTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29775
Application Number = 19777
Date of Application = 19,, May, 1988
RX/OTC/DISCN = RX
Tradename = ZESTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 29776
Application Number = 19777
Date of Application = 19,, May, 1988
RX/OTC/DISCN = RX
Tradename = ZESTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 29778
Application Number = 19777
Date of Application = 19,, May, 1988
RX/OTC/DISCN = RX
Tradename = ZESTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 40MG
----
Product id = 29773
Application Number = 19777
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = RX
Tradename = ZESTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 005
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 29777
Application Number = 19777
Date of Application = 20,, Jan, 1999
RX/OTC/DISCN = RX
Tradename = ZESTRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 006
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 26694
Application Number = 19778
Date of Application = 16,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = PRINZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 26695
Application Number = 19778
Date of Application = 16,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = PRINZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;20MG
----
Product id = 26693
Application Number = 19778
Date of Application = 18,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = PRINZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 29771
Application Number = 19888
Date of Application = 20,, Sep, 1990
RX/OTC/DISCN = RX
Tradename = ZESTORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 12.5MG;20MG
----
Product id = 29772
Application Number = 19888
Date of Application = 20,, Jul, 1989
RX/OTC/DISCN = RX
Tradename = ZESTORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;20MG
----
Product id = 29770
Application Number = 19888
Date of Application = 18,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = ZESTORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23776
Application Number = 75743
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23777
Application Number = 75743
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23778
Application Number = 75743
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23779
Application Number = 75743
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23780
Application Number = 75743
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23781
Application Number = 75743
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23722
Application Number = 75752
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23723
Application Number = 75752
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23724
Application Number = 75752
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23725
Application Number = 75752
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23726
Application Number = 75752
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23727
Application Number = 75752
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23803
Application Number = 75776
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23804
Application Number = 75776
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23805
Application Number = 75776
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23770
Application Number = 75783
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 23771
Application Number = 75783
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23772
Application Number = 75783
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23773
Application Number = 75783
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode =
Rld = No
Strength = 20MG
----
Product id = 23774
Application Number = 75783
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 005
Tecode =
Rld = No
Strength = 30MG
----
Product id = 23775
Application Number = 75783
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 006
Tecode =
Rld = No
Strength = 40MG
----
Product id = 23824
Application Number = 75869
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23825
Application Number = 75869
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23826
Application Number = 75869
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;20MG
----
Product id = 23752
Application Number = 75903
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 23755
Application Number = 75903
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23758
Application Number = 75903
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23761
Application Number = 75903
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 20MG
----
Product id = 23764
Application Number = 75903
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode =
Rld = No
Strength = 30MG
----
Product id = 23767
Application Number = 75903
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 006
Tecode =
Rld = No
Strength = 40MG
----
Product id = 23818
Application Number = 75926
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23820
Application Number = 75926
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23822
Application Number = 75926
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;20MG
----
Product id = 23746
Application Number = 75944
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23747
Application Number = 75944
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23748
Application Number = 75944
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23749
Application Number = 75944
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23751
Application Number = 75944
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 005
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23750
Application Number = 75944
Date of Application = 11,, Feb, 2003
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 006
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23753
Application Number = 75994
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23756
Application Number = 75994
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23759
Application Number = 75994
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23762
Application Number = 75994
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23765
Application Number = 75994
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23768
Application Number = 75994
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23754
Application Number = 75999
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 23757
Application Number = 75999
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23760
Application Number = 75999
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23763
Application Number = 75999
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 20MG
----
Product id = 23766
Application Number = 75999
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode =
Rld = No
Strength = 30MG
----
Product id = 23769
Application Number = 75999
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 006
Tecode =
Rld = No
Strength = 40MG
----
Product id = 23815
Application Number = 76007
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23816
Application Number = 76007
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23817
Application Number = 76007
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23782
Application Number = 76059
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23783
Application Number = 76059
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23784
Application Number = 76059
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23785
Application Number = 76059
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23786
Application Number = 76059
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23787
Application Number = 76059
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23710
Application Number = 76063
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23711
Application Number = 76063
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23712
Application Number = 76063
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23713
Application Number = 76063
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23715
Application Number = 76063
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23714
Application Number = 76063
Date of Application = 27,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 006
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23734
Application Number = 76071
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23735
Application Number = 76071
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23736
Application Number = 76071
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23737
Application Number = 76071
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23738
Application Number = 76071
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23739
Application Number = 76071
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23698
Application Number = 76102
Date of Application = 30,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23699
Application Number = 76102
Date of Application = 30,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23700
Application Number = 76102
Date of Application = 30,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23701
Application Number = 76102
Date of Application = 30,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23702
Application Number = 76102
Date of Application = 30,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23703
Application Number = 76102
Date of Application = 30,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23809
Application Number = 76113
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23810
Application Number = 76113
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23811
Application Number = 76113
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23743
Application Number = 76164
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23744
Application Number = 76164
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23745
Application Number = 76164
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23740
Application Number = 76180
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23741
Application Number = 76180
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23742
Application Number = 76180
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23828
Application Number = 76194
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23829
Application Number = 76194
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23827
Application Number = 76194
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23812
Application Number = 76230
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23813
Application Number = 76230
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23814
Application Number = 76230
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23819
Application Number = 76262
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23821
Application Number = 76262
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23823
Application Number = 76262
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23800
Application Number = 76265
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23801
Application Number = 76265
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23802
Application Number = 76265
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23794
Application Number = 76674
Date of Application = 5,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23795
Application Number = 76674
Date of Application = 5,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23796
Application Number = 76674
Date of Application = 5,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23728
Application Number = 77321
Date of Application = 9,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23729
Application Number = 77321
Date of Application = 9,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23730
Application Number = 77321
Date of Application = 9,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23731
Application Number = 77321
Date of Application = 9,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23732
Application Number = 77321
Date of Application = 9,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23733
Application Number = 77321
Date of Application = 9,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23797
Application Number = 77606
Date of Application = 14,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23798
Application Number = 77606
Date of Application = 14,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23799
Application Number = 77606
Date of Application = 14,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23704
Application Number = 77622
Date of Application = 22,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23705
Application Number = 77622
Date of Application = 22,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23706
Application Number = 77622
Date of Application = 22,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23707
Application Number = 77622
Date of Application = 22,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23708
Application Number = 77622
Date of Application = 22,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23709
Application Number = 77622
Date of Application = 22,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23806
Application Number = 77912
Date of Application = 27,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23807
Application Number = 77912
Date of Application = 27,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23808
Application Number = 77912
Date of Application = 27,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23788
Application Number = 78402
Date of Application = 19,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23789
Application Number = 78402
Date of Application = 19,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23790
Application Number = 78402
Date of Application = 19,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23791
Application Number = 78402
Date of Application = 19,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23792
Application Number = 78402
Date of Application = 19,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23793
Application Number = 78402
Date of Application = 19,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23692
Application Number = 202554
Date of Application = 30,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23693
Application Number = 202554
Date of Application = 30,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23694
Application Number = 202554
Date of Application = 30,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23695
Application Number = 202554
Date of Application = 30,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23696
Application Number = 202554
Date of Application = 30,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23697
Application Number = 202554
Date of Application = 30,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 23716
Application Number = 203508
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23717
Application Number = 203508
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23718
Application Number = 203508
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23719
Application Number = 203508
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23720
Application Number = 203508
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 23721
Application Number = 203508
Date of Application = 29,, Oct, 2013
RX/OTC/DISCN = RX
Tradename = LISINOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 006
Tecode = AB
Rld = No
Strength = 40MG
----