letrozole
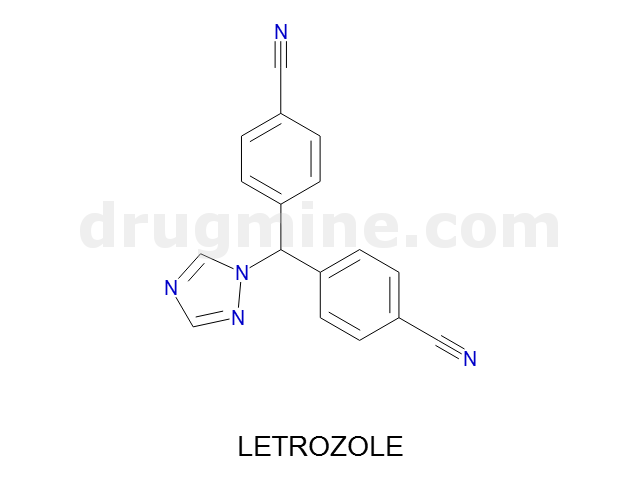
Name: LETROZOLE
ID :
MW: 285
Number of atoms: 22
Molecular_Formula: C17H11N5
Alogp: 2.749
Indication class : Antineoplastic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
letrozole containing products summary
There are in total 18 different products containing the active ingredient letrozole. From the 18 drug products, 3 have been discontinued.Product id = 21228
Application Number = 20726
Date of Application = 25,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = FEMARA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 2.5MG
----
Product id = 23383
Application Number = 78190
Date of Application = 24,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23387
Application Number = 90196
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = DISCN
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 23388
Application Number = 90289
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23374
Application Number = 90292
Date of Application = 13,, Jul, 2011
RX/OTC/DISCN = DISCN
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS TOTOWA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 23377
Application Number = 90491
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = FRESENIUS KABI ONCOL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23389
Application Number = 90789
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23385
Application Number = 90838
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23373
Application Number = 90934
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23381
Application Number = 91098
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23376
Application Number = 91191
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23375
Application Number = 91303
Date of Application = 19,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23386
Application Number = 91466
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23378
Application Number = 91638
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = DISCN
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 23384
Application Number = 200161
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NATCO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23379
Application Number = 201804
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INDICUS PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23382
Application Number = 202048
Date of Application = 29,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT HOLDINGS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 23380
Application Number = 202716
Date of Application = 16,, May, 2013
RX/OTC/DISCN = RX
Tradename = LETROZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JIANGSU HENGRUI MED
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----