ketoconazole
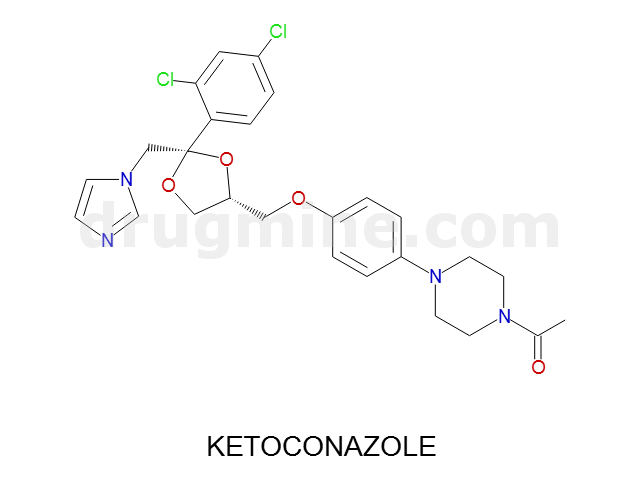

Name: KETOCONAZOLE
ID :
MW: 531
Number of atoms: 36
Molecular_Formula: C26H28Cl2N4O4
Alogp: 3.61
Indication class : Antifungal
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ketoconazole containing products summary
There are in total 21 different products containing the active ingredient ketoconazole. From the 21 drug products, 8 have been discontinued.Product id = 25454
Application Number = 18533
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NIZORAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 4340
Application Number = 19084
Date of Application = 31,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = NIZORAL
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = JANSSEN PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 2%
----
Product id = 12826
Application Number = 19927
Date of Application = 31,, Aug, 1990
RX/OTC/DISCN = RX
Tradename = NIZORAL
Route/format = TOPICAL / SHAMPOO
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 2%
----
Product id = 12827
Application Number = 20310
Date of Application = 10,, Oct, 1997
RX/OTC/DISCN = OTC
Tradename = NIZORAL A-D
Route/format = TOPICAL / SHAMPOO
Application Type = N
Applicant Name = JOHNSON AND JOHNSON
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1%
----
Product id = 8
Application Number = 21738
Date of Application = 12,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = EXTINA
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = N
Applicant Name = DELCOR ASSET CORP
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 2%
----
Product id = 5448
Application Number = 21946
Date of Application = 28,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = XOLEGEL
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = AQUA PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2%
----
Product id = 14758
Application Number = 70767
Date of Application = 7,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = NIZORAL
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = JANSSEN PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 23173
Application Number = 74971
Date of Application = 15,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 23174
Application Number = 75273
Date of Application = 15,, Jun, 1999
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 23169
Application Number = 75314
Date of Application = 15,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 23172
Application Number = 75319
Date of Application = 15,, Jun, 1999
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 23167
Application Number = 75341
Date of Application = 27,, Jul, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AAIPHARMA LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 23171
Application Number = 75362
Date of Application = 15,, Jun, 1999
RX/OTC/DISCN = DISCN
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 4280
Application Number = 75581
Date of Application = 25,, Apr, 2000
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 2%
----
Product id = 23170
Application Number = 75597
Date of Application = 23,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 4281
Application Number = 75638
Date of Application = 18,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = KETOZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2%
----
Product id = 23168
Application Number = 75912
Date of Application = 10,, Jan, 2002
RX/OTC/DISCN = DISCN
Tradename = KETOCONAZOLE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 4279
Application Number = 76294
Date of Application = 28,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2%
----
Product id = 12819
Application Number = 76419
Date of Application = 7,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2%
----
Product id = 12820
Application Number = 76942
Date of Application = 11,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2%
----
Product id = 10
Application Number = 91550
Date of Application = 25,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = KETOCONAZOLE
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AT
Rld = No
Strength = 2%
----