ketamine-hydrochloride
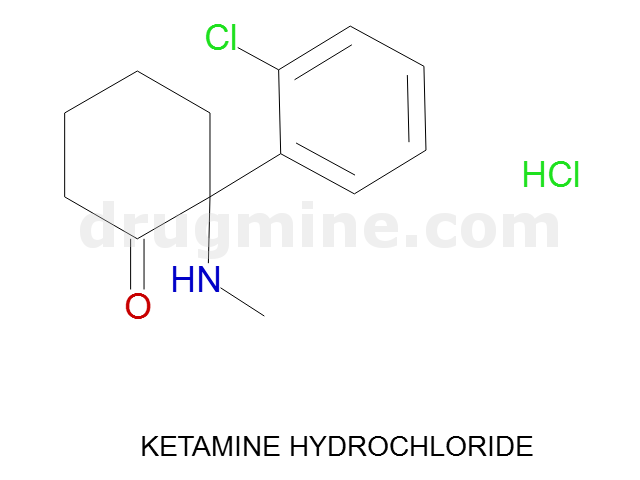
Name: KETAMINE HYDROCHLORIDE
ID :
MW: 238
Number of atoms: 16
Molecular_Formula: C13H16ClNO
Alogp: 2.88
Indication class : Anesthetic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
ketamine hydrochloride containing products summary
There are in total 10 different products containing the active ingredient ketamine hydrochloride. From the 10 drug products, zero have been discontinued.Product id = 8607
Application Number = 16812
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KETALAR
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PAR STERILE PRODUCTS
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 10MG BASE/ML
----
Product id = 8608
Application Number = 16812
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KETALAR
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PAR STERILE PRODUCTS
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 50MG BASE/ML
----
Product id = 8609
Application Number = 16812
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KETALAR
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PAR STERILE PRODUCTS
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 100MG BASE/ML
----
Product id = 8610
Application Number = 74524
Date of Application = 22,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = KETAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG BASE/ML
----
Product id = 8611
Application Number = 74524
Date of Application = 22,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = KETAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 100MG BASE/ML
----
Product id = 8612
Application Number = 74549
Date of Application = 27,, Jun, 1996
RX/OTC/DISCN = RX
Tradename = KETAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 50MG BASE/ML
----
Product id = 8613
Application Number = 74549
Date of Application = 27,, Jun, 1996
RX/OTC/DISCN = RX
Tradename = KETAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 100MG BASE/ML
----
Product id = 8614
Application Number = 76092
Date of Application = 30,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = KETAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 8615
Application Number = 76092
Date of Application = 28,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = KETAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 50MG BASE/ML
----
Product id = 8616
Application Number = 76092
Date of Application = 25,, Oct, 2002
RX/OTC/DISCN = RX
Tradename = KETAMINE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 100MG BASE/ML
----