isosorbide-mononitrate
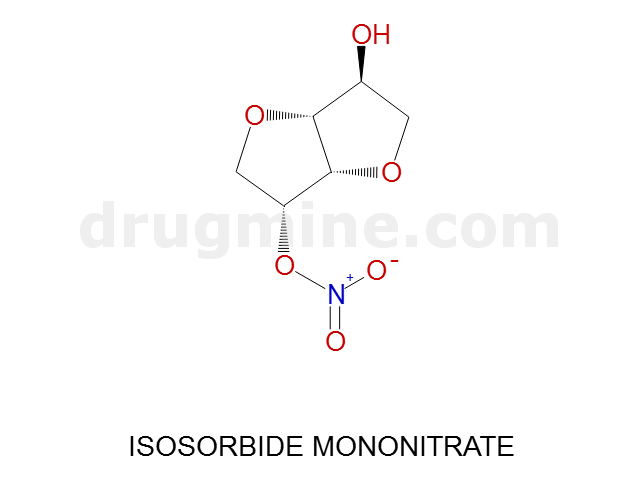
Name: ISOSORBIDE MONONITRATE
ID :
MW: 191
Number of atoms: 13
Molecular_Formula: C6H9NO6
Alogp: 1.003
Indication class : Vasodilator (coronary)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
isosorbide mononitrate containing products summary
There are in total 32 different products containing the active ingredient isosorbide mononitrate. From the 32 drug products, 11 have been discontinued.Product id = 23029
Application Number = 19091
Date of Application = 30,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = ISMO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PROMIUS PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 25067
Application Number = 20215
Date of Application = 30,, Jun, 1993
RX/OTC/DISCN = RX
Tradename = MONOKET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KREMERS URBAN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 20MG
----
Product id = 25066
Application Number = 20215
Date of Application = 30,, Jun, 1993
RX/OTC/DISCN = RX
Tradename = MONOKET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = KREMERS URBAN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 15995
Application Number = 20225
Date of Application = 12,, Aug, 1993
RX/OTC/DISCN = DISCN
Tradename = IMDUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 15996
Application Number = 20225
Date of Application = 12,, Aug, 1993
RX/OTC/DISCN = DISCN
Tradename = IMDUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 002
Tecode =
Rld = No
Strength = 60MG
----
Product id = 15997
Application Number = 20225
Date of Application = 30,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = IMDUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHERING PLOUGH
ProductNo = 003
Tecode =
Rld = No
Strength = 120MG
----
Product id = 23098
Application Number = 75037
Date of Application = 30,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 23097
Application Number = 75037
Date of Application = 30,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 16012
Application Number = 75041
Date of Application = 22,, Sep, 1998
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ALKERMES GAINESVILLE
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 23099
Application Number = 75147
Date of Application = 27,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 16020
Application Number = 75155
Date of Application = 30,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KREMERS URBAN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 16019
Application Number = 75155
Date of Application = 13,, Jan, 2000
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KREMERS URBAN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 16021
Application Number = 75155
Date of Application = 4,, Aug, 2000
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = KREMERS URBAN PHARMS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 120MG
----
Product id = 16025
Application Number = 75166
Date of Application = 7,, Oct, 1999
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SKYEPHARMA AG
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 16010
Application Number = 75306
Date of Application = 31,, Dec, 1998
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 16011
Application Number = 75306
Date of Application = 31,, Dec, 1998
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 60MG
----
Product id = 23100
Application Number = 75361
Date of Application = 5,, Oct, 2000
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 16022
Application Number = 75395
Date of Application = 16,, Mar, 2000
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 16023
Application Number = 75395
Date of Application = 16,, Mar, 2000
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 16024
Application Number = 75395
Date of Application = 16,, Mar, 2000
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 16017
Application Number = 75448
Date of Application = 19,, Jun, 2000
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 60MG
----
Product id = 16016
Application Number = 75448
Date of Application = 7,, Aug, 2001
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 30MG
----
Product id = 16018
Application Number = 75448
Date of Application = 7,, Aug, 2001
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 120MG
----
Product id = 16013
Application Number = 75522
Date of Application = 17,, Apr, 2000
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DEXCEL LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 16015
Application Number = 76813
Date of Application = 7,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 16014
Application Number = 76813
Date of Application = 30,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = HIKMA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 16029
Application Number = 90598
Date of Application = 11,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 16030
Application Number = 90598
Date of Application = 11,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 16031
Application Number = 90598
Date of Application = 11,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 120MG
----
Product id = 16026
Application Number = 200270
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 16027
Application Number = 200495
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 60MG
----
Product id = 16028
Application Number = 200495
Date of Application = 3,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE MONONITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 120MG
----