isosorbide-dinitrate
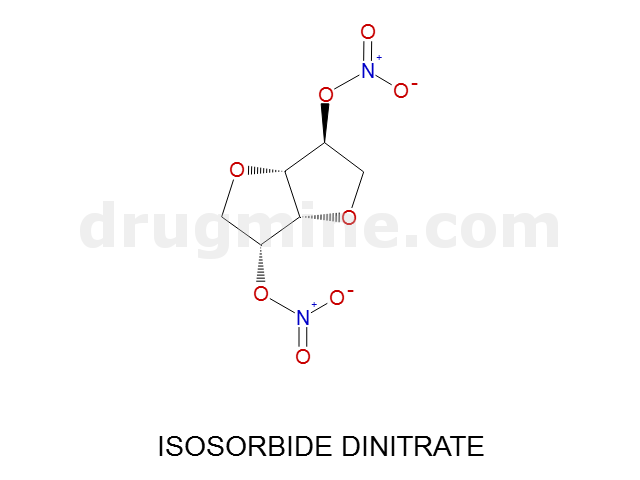

Name: ISOSORBIDE DINITRATE
ID :
MW: 236
Number of atoms: 16
Molecular_Formula: C6H8N2O8
Alogp: 3.413
Indication class : Vasodilator (coronary)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
isosorbide dinitrate containing products summary
There are in total 52 different products containing the active ingredient isosorbide dinitrate. From the 52 drug products, 35 have been discontinued.Product id = 23076
Application Number = 12093
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = ISORDIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 001
Tecode =
Rld = Yes
Strength = 40MG
----
Product id = 23073
Application Number = 12093
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23075
Application Number = 12093
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 005
Tecode =
Rld = No
Strength = 30MG
----
Product id = 23074
Application Number = 12093
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 006
Tecode =
Rld = No
Strength = 20MG
----
Product id = 23072
Application Number = 12093
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = ISORDIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT PHARMS NORTH
ProductNo = 007
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 16007
Application Number = 12882
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 568
Application Number = 12882
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 002
Tecode =
Rld = No
Strength = 40MG
----
Product id = 30195
Application Number = 12940
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = BIOVAIL
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG
----
Product id = 30194
Application Number = 12940
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = BIOVAIL
ProductNo = 004
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 30196
Application Number = 12940
Date of Application = 29,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = ISORDIL
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = BIOVAIL
ProductNo = 005
Tecode =
Rld = No
Strength = 10MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 30212
Application Number = 16191
Date of Application = 1,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 30211
Application Number = 16191
Date of Application = 1,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 28103
Application Number = 16192
Date of Application = 1,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 28104
Application Number = 16192
Date of Application = 1,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 15362
Application Number = 16776
Date of Application = 1,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 15363
Application Number = 16776
Date of Application = 1,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 431
Application Number = 19790
Date of Application = 2,, Sep, 1988
RX/OTC/DISCN = RX
Tradename = DILATRATE-SR
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = AUXILIUM PHARMS LLC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 40MG
----
Product id = 18564
Application Number = 20727
Date of Application = 23,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = BIDIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ARBOR PHARMS LLC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 37.5MG;20MG
----
Product id = 16009
Application Number = 40009
Date of Application = 30,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 40MG
----
Product id = 23080
Application Number = 40591
Date of Application = 10,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 16008
Application Number = 40723
Date of Application = 17,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = COREPHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 30199
Application Number = 84204
Date of Application = 18,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 30205
Application Number = 86031
Date of Application = 29,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 23096
Application Number = 86032
Date of Application = 7,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 30204
Application Number = 86033
Date of Application = 26,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 23095
Application Number = 86034
Date of Application = 6,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 30197
Application Number = 86054
Date of Application = 29,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 30198
Application Number = 86055
Date of Application = 2,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23078
Application Number = 86066
Date of Application = 29,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23077
Application Number = 86067
Date of Application = 29,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23081
Application Number = 86166
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23083
Application Number = 86167
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 30200
Application Number = 86168
Date of Application = 18,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23082
Application Number = 86169
Date of Application = 19,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23089
Application Number = 86221
Date of Application = 7,, Jan, 1988
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 30203
Application Number = 86222
Date of Application = 19,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23090
Application Number = 86223
Date of Application = 7,, Jan, 1988
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 30202
Application Number = 86225
Date of Application = 19,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 28105
Application Number = 86405
Date of Application = 21,, Aug, 1990
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 23085
Application Number = 86923
Date of Application = 12,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 23086
Application Number = 86925
Date of Application = 12,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 23087
Application Number = 87537
Date of Application = 2,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 30201
Application Number = 87545
Date of Application = 18,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23084
Application Number = 87564
Date of Application = 18,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 23088
Application Number = 87946
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 30MG
----
Product id = 23079
Application Number = 88088
Date of Application = 2,, Nov, 1987
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28106
Application Number = 88124
Date of Application = 21,, Aug, 1990
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG
----
Product id = 28107
Application Number = 88125
Date of Application = 21,, Aug, 1990
RX/OTC/DISCN = DISCN
Tradename = SORBITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 40MG
----
Product id = 23092
Application Number = 89190
Date of Application = 17,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 23093
Application Number = 89191
Date of Application = 17,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 23094
Application Number = 89192
Date of Application = 17,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG
----
Product id = 23091
Application Number = 89367
Date of Application = 7,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = ISOSORBIDE DINITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----