ifosfamide
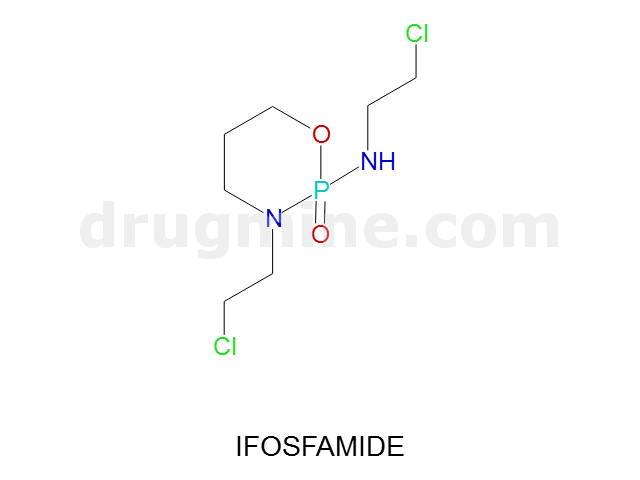
Name: IFOSFAMIDE
ID :
MW: 261
Number of atoms: 14
Molecular_Formula: C7H15Cl2N2O2P
Alogp: 0.144
Indication class : Antineoplastic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
ifosfamide containing products summary
There are in total 16 different products containing the active ingredient ifosfamide. From the 16 drug products, 2 have been discontinued.Product id = 8377
Application Number = 19763
Date of Application = 30,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = IFEX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1GM/VIAL
----
Product id = 8378
Application Number = 19763
Date of Application = 30,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = IFEX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode = AP
Rld = No
Strength = 3GM/VIAL
----
Product id = 8379
Application Number = 19763
Date of Application = 10,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = IFEX/MESNEX KIT
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM/VIAL;100MG/ML
----
Product id = 8380
Application Number = 19763
Date of Application = 10,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = IFEX/MESNEX KIT
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode =
Rld = No
Strength = 3GM/VIAL;100MG/ML
----
Product id = 11688
Application Number = 75874
Date of Application = 26,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE/MESNA KIT
Route/format = INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1GM/20ML;1GM/10ML (50MG/ML;100MG/ML)
----
Product id = 11689
Application Number = 75874
Date of Application = 26,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE/MESNA KIT
Route/format = INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode =
Rld = Yes
Strength = 3GM/60ML;1GM/10ML (50MG/ML;100MG/ML)
----
Product id = 8383
Application Number = 76078
Date of Application = 28,, May, 2002
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 1GM/VIAL
----
Product id = 8385
Application Number = 76078
Date of Application = 28,, May, 2002
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 3GM/VIAL
----
Product id = 8381
Application Number = 76619
Date of Application = 29,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1GM/20ML(50MG/ML)
----
Product id = 8382
Application Number = 76619
Date of Application = 29,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 002
Tecode = AP
Rld = No
Strength = 3GM/60ML(50MG/ML)
----
Product id = 8389
Application Number = 76657
Date of Application = 4,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 1GM/20ML (50MG/ML)
----
Product id = 8390
Application Number = 76657
Date of Application = 4,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 3GM/60ML (50MG/ML)
----
Product id = 8384
Application Number = 90181
Date of Application = 22,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1GM/20ML (50MG/ML)
----
Product id = 8386
Application Number = 90181
Date of Application = 22,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 3GM/60ML (50MG/ML)
----
Product id = 8387
Application Number = 201689
Date of Application = 26,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1GM/20ML (50MG/ML)
----
Product id = 8388
Application Number = 201689
Date of Application = 26,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = IFOSFAMIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 002
Tecode = AP
Rld = No
Strength = 3GM/60ML (50MG/ML)
----