hydroxychloroquine-sulfate
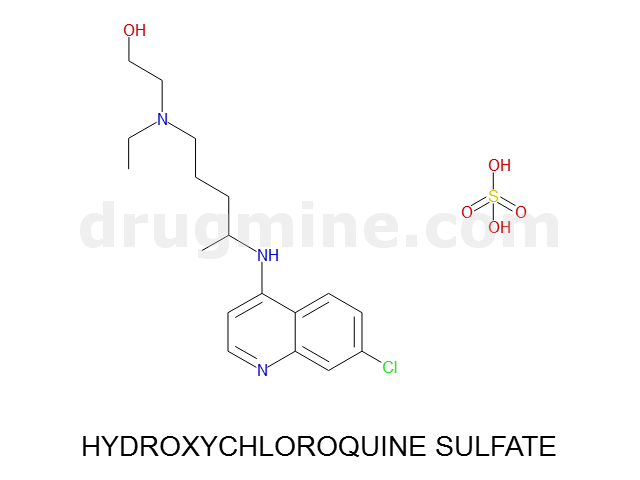
Name: HYDROXYCHLOROQUINE SULFATE
ID :
MW: 336
Number of atoms: 23
Molecular_Formula: C18H26ClN3O
Alogp: 3.457
Indication class : Antimalarial; Suppressant (lupus erythematosus)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
hydroxychloroquine sulfate containing products summary
There are in total 9 different products containing the active ingredient hydroxychloroquine sulfate. From the 9 drug products, 2 have been discontinued.Product id = 26313
Application Number = 9768
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PLAQUENIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 22581
Application Number = 40081
Date of Application = 30,, Sep, 1994
RX/OTC/DISCN = RX
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 22579
Application Number = 40104
Date of Application = 30,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 22582
Application Number = 40133
Date of Application = 30,, Nov, 1995
RX/OTC/DISCN = DISCN
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22580
Application Number = 40150
Date of Application = 27,, Jan, 1996
RX/OTC/DISCN = DISCN
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 22578
Application Number = 40274
Date of Application = 29,, May, 1998
RX/OTC/DISCN = RX
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 22583
Application Number = 40657
Date of Application = 21,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 22576
Application Number = 40760
Date of Application = 15,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 22577
Application Number = 40766
Date of Application = 14,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = HYDROXYCHLOROQUINE SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----