hydromorphone-hydrochloride
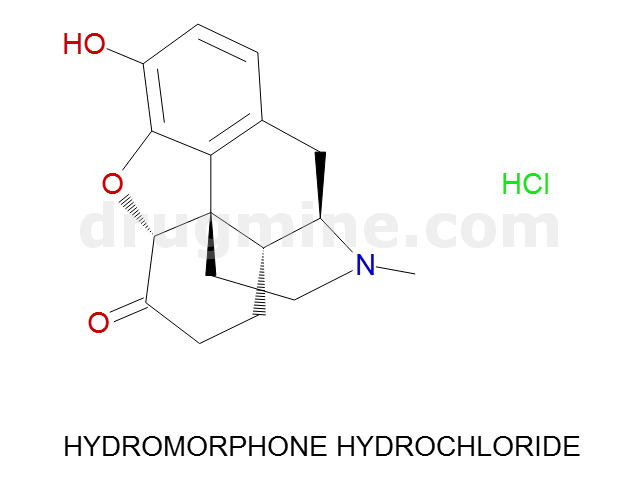
Name: HYDROMORPHONE HYDROCHLORIDE
ID :
MW: 285
Number of atoms: 21
Molecular_Formula: C17H19NO3
Alogp: 1.715
Indication class : Analgesic (narcotic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
hydromorphone hydrochloride containing products summary
There are in total 42 different products containing the active ingredient hydromorphone hydrochloride. From the 42 drug products, 8 have been discontinued.Product id = 7240
Application Number = 19034
Date of Application = 11,, Jan, 1984
RX/OTC/DISCN = RX
Tradename = DILAUDID-HP
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 10MG/ML
----
Product id = 7241
Application Number = 19034
Date of Application = 4,, Aug, 1994
RX/OTC/DISCN = RX
Tradename = DILAUDID-HP
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 250MG/VIAL
----
Product id = 7237
Application Number = 19034
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = DILAUDID
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 1MG/ML
----
Product id = 7238
Application Number = 19034
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = DILAUDID
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = 2MG/ML
----
Product id = 7239
Application Number = 19034
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = DILAUDID
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 005
Tecode = AP
Rld = Yes
Strength = 4MG/ML
----
Product id = 13794
Application Number = 19891
Date of Application = 7,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DILAUDID
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 5MG/5ML
----
Product id = 20460
Application Number = 19892
Date of Application = 7,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = DILAUDID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 8MG
----
Product id = 20459
Application Number = 19892
Date of Application = 9,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = DILAUDID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 20458
Application Number = 19892
Date of Application = 9,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = DILAUDID
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PURDUE PHARM PRODS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 654
Application Number = 21044
Date of Application = 24,, Sep, 2004
RX/OTC/DISCN = DISCN
Tradename = PALLADONE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA LP
ProductNo = 001
Tecode =
Rld = No
Strength = 12MG
----
Product id = 655
Application Number = 21044
Date of Application = 24,, Sep, 2004
RX/OTC/DISCN = DISCN
Tradename = PALLADONE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA LP
ProductNo = 002
Tecode =
Rld = No
Strength = 16MG
----
Product id = 656
Application Number = 21044
Date of Application = 24,, Sep, 2004
RX/OTC/DISCN = DISCN
Tradename = PALLADONE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA LP
ProductNo = 003
Tecode =
Rld = No
Strength = 24MG
----
Product id = 657
Application Number = 21044
Date of Application = 24,, Sep, 2004
RX/OTC/DISCN = DISCN
Tradename = PALLADONE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA LP
ProductNo = 004
Tecode =
Rld = No
Strength = 32MG
----
Product id = 15926
Application Number = 21217
Date of Application = 1,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = EXALGO
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 15927
Application Number = 21217
Date of Application = 1,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = EXALGO
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12MG
----
Product id = 15928
Application Number = 21217
Date of Application = 1,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = EXALGO
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 16MG
----
Product id = 15929
Application Number = 21217
Date of Application = 24,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = EXALGO
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MALLINCKRODT INC
ProductNo = 004
Tecode =
Rld = Yes
Strength = 32MG
----
Product id = 8307
Application Number = 74317
Date of Application = 23,, Aug, 1995
RX/OTC/DISCN = DISCN
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG/ML
----
Product id = 22570
Application Number = 74597
Date of Application = 29,, Jul, 1998
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 22569
Application Number = 74597
Date of Application = 29,, May, 2009
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 8302
Application Number = 74598
Date of Application = 19,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 13856
Application Number = 74653
Date of Application = 29,, Jul, 1998
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = No
Strength = 5MG/5ML
----
Product id = 8301
Application Number = 76444
Date of Application = 25,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 22559
Application Number = 76723
Date of Application = 18,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ELITE LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 22563
Application Number = 76855
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 22566
Application Number = 77311
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = DISCN
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 22567
Application Number = 77311
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = DISCN
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG
----
Product id = 22568
Application Number = 77311
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = DISCN
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 8MG
----
Product id = 22562
Application Number = 77471
Date of Application = 9,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 8299
Application Number = 78228
Date of Application = 14,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 8300
Application Number = 78261
Date of Application = 14,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 22564
Application Number = 78273
Date of Application = 19,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 22565
Application Number = 78273
Date of Application = 19,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MALLINCKRODT INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 22560
Application Number = 78439
Date of Application = 9,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 22561
Application Number = 78439
Date of Application = 9,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 8306
Application Number = 78591
Date of Application = 17,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = 10MG/ML
----
Product id = 8303
Application Number = 200403
Date of Application = 1,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 8304
Application Number = 200403
Date of Application = 1,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 8305
Application Number = 200403
Date of Application = 1,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 003
Tecode = AP
Rld = No
Strength = 4MG/ML
----
Product id = 15985
Application Number = 202144
Date of Application = 12,, May, 2014
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 15986
Application Number = 202144
Date of Application = 12,, May, 2014
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12MG
----
Product id = 15987
Application Number = 202144
Date of Application = 12,, May, 2014
RX/OTC/DISCN = RX
Tradename = HYDROMORPHONE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 16MG
----