hydroflumethiazide
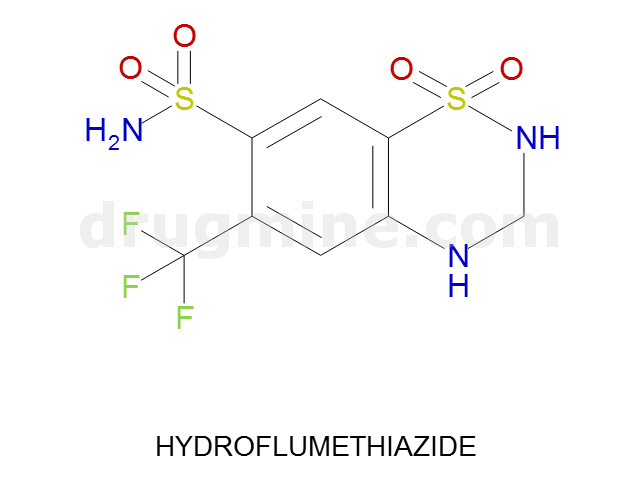
Name: HYDROFLUMETHIAZIDE
ID :
MW: 331
Number of atoms: 20
Molecular_Formula: C8H8F3N3O4S2
Alogp: -2.e-003
Indication class : Antihypertensive; Diuretic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
hydroflumethiazide containing products summary
There are in total 12 different products containing the active ingredient hydroflumethiazide. From the 12 drug products, 11 have been discontinued.Product id = 27821
Application Number = 11949
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SALURON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE LLC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 50MG
----
Product id = 27822
Application Number = 12359
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SALUTENSIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27823
Application Number = 12359
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SALUTENSIN-DEMI
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SHIRE
ProductNo = 004
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 20569
Application Number = 83383
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIUCARDIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22554
Application Number = 88031
Date of Application = 6,, Apr, 1983
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22558
Application Number = 88110
Date of Application = 22,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22557
Application Number = 88127
Date of Application = 22,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22556
Application Number = 88195
Date of Application = 26,, Oct, 1983
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22555
Application Number = 88528
Date of Application = 15,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22553
Application Number = 88850
Date of Application = 31,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = HYDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 27504
Application Number = 88907
Date of Application = 20,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27503
Application Number = 88932
Date of Application = 11,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROFLUMETHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----