hydrochlorothiazide
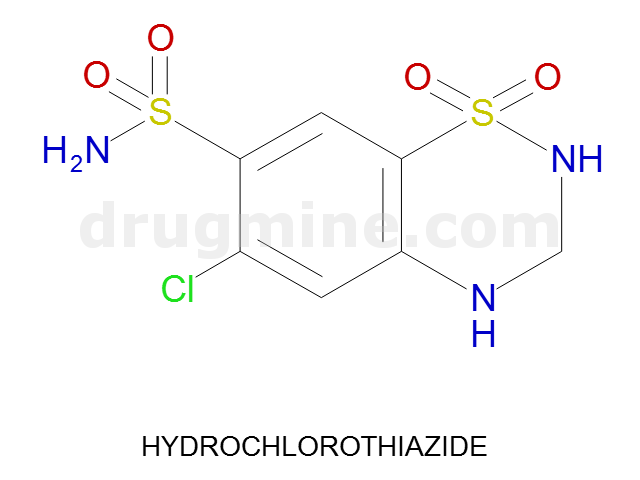
Name: HYDROCHLOROTHIAZIDE
ID :
MW: 298
Number of atoms: 17
Molecular_Formula: C7H8ClN3O4S2
Alogp: -0.28
Indication class : Diuretic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
hydrochlorothiazide containing products summary
There are in total 710 different products containing the active ingredient hydrochlorothiazide. From the 710 drug products, 309 have been discontinued.Product id = 20976
Application Number = 11793
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ESIDRIX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 25MG
----
Product id = 20977
Application Number = 11793
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ESIDRIX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 008
Tecode =
Rld = No
Strength = 50MG
----
Product id = 20978
Application Number = 11793
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ESIDRIX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 009
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22550
Application Number = 11835
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRODIURIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22551
Application Number = 11835
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRODIURIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 006
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22552
Application Number = 11835
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRODIURIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 007
Tecode =
Rld = No
Strength = 100MG
----
Product id = 27895
Application Number = 11878
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL-ESIDRIX #1
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;0.1MG
----
Product id = 27896
Application Number = 11878
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SERPASIL-ESIDRIX #2
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 50MG;0.1MG
----
Product id = 22573
Application Number = 11958
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROPRES 25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22574
Application Number = 11958
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROPRES 50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 25661
Application Number = 11971
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 25662
Application Number = 11971
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = ORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18051
Application Number = 12026
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = APRESOLINE-ESIDRIX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 25663
Application Number = 12148
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ORETICYL 25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.125MG;25MG
----
Product id = 25665
Application Number = 12148
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ORETICYL FORTE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode =
Rld = No
Strength = 0.25MG;25MG
----
Product id = 25664
Application Number = 12148
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ORETICYL 50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 003
Tecode =
Rld = No
Strength = 0.125MG;50MG
----
Product id = 27871
Application Number = 12193
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SER-AP-ES
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 17426
Application Number = 12616
Date of Application = 30,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = ALDACTAZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 25MG;25MG
----
Product id = 17427
Application Number = 12616
Date of Application = 30,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = ALDACTAZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GD SEARLE LLC
ProductNo = 005
Tecode =
Rld = Yes
Strength = 50MG;50MG
----
Product id = 17436
Application Number = 13402
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALDORIL 15
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 17437
Application Number = 13402
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALDORIL 25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 17438
Application Number = 13402
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALDORIL D30
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 17439
Application Number = 13402
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ALDORIL D50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 20979
Application Number = 13553
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ESIMIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;25MG
----
Product id = 1824
Application Number = 16042
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DYAZIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;50MG
----
Product id = 1823
Application Number = 16042
Date of Application = 3,, Mar, 1994
RX/OTC/DISCN = RX
Tradename = DYAZIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = GLAXOSMITHKLINE LLC
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 25MG;37.5MG
----
Product id = 22925
Application Number = 18031
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = INDERIDE-40/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG;40MG
----
Product id = 22926
Application Number = 18031
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = INDERIDE-80/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = WYETH PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 28716
Application Number = 18061
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TIMOLIDE 10-25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;10MG
----
Product id = 25044
Application Number = 18201
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MODURETIC 5-50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG ANHYDROUS;50MG
----
Product id = 23861
Application Number = 18303
Date of Application = 31,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = LOPRESSOR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 23862
Application Number = 18303
Date of Application = 31,, Dec, 1984
RX/OTC/DISCN = RX
Tradename = LOPRESSOR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;100MG
----
Product id = 23863
Application Number = 18303
Date of Application = 31,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = LOPRESSOR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;100MG
----
Product id = 18836
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 25/15
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18837
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 25/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;25MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18839
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 50/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;25MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18838
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 50/15
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG;15MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 29601
Application Number = 18872
Date of Application = 22,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = VISKAZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;5MG
----
Product id = 29602
Application Number = 18872
Date of Application = 22,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = VISKAZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;10MG
----
Product id = 25481
Application Number = 19046
Date of Application = 6,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = NORMOZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 25482
Application Number = 19046
Date of Application = 6,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = NORMOZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;200MG
----
Product id = 25483
Application Number = 19046
Date of Application = 6,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = NORMOZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;300MG
----
Product id = 25484
Application Number = 19046
Date of Application = 6,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = NORMOZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 004
Tecode =
Rld = No
Strength = 25MG;400MG
----
Product id = 552
Application Number = 19059
Date of Application = 3,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = INDERIDE LA 80/50
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;80MG
----
Product id = 550
Application Number = 19059
Date of Application = 3,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = INDERIDE LA 120/50
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG;120MG
----
Product id = 551
Application Number = 19059
Date of Application = 3,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = INDERIDE LA 160/50
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;160MG
----
Product id = 24131
Application Number = 19129
Date of Application = 22,, Oct, 1984
RX/OTC/DISCN = RX
Tradename = MAXZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 50MG;75MG
----
Product id = 24132
Application Number = 19129
Date of Application = 13,, May, 1988
RX/OTC/DISCN = RX
Tradename = MAXZIDE-25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 29016
Application Number = 19174
Date of Application = 10,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = TRANDATE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 29017
Application Number = 19174
Date of Application = 10,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = TRANDATE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;200MG
----
Product id = 29018
Application Number = 19174
Date of Application = 10,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = TRANDATE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;300MG
----
Product id = 29019
Application Number = 19174
Date of Application = 10,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = TRANDATE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 004
Tecode =
Rld = No
Strength = 25MG;400MG
----
Product id = 29457
Application Number = 19221
Date of Application = 31,, Oct, 1986
RX/OTC/DISCN = RX
Tradename = VASERETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 10MG;25MG
----
Product id = 29456
Application Number = 19221
Date of Application = 12,, Jul, 1995
RX/OTC/DISCN = RX
Tradename = VASERETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = VALEANT INTL
ProductNo = 003
Tecode = AB
Rld = No
Strength = 5MG;12.5MG
----
Product id = 26694
Application Number = 19778
Date of Application = 16,, Feb, 1989
RX/OTC/DISCN = RX
Tradename = PRINZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 26695
Application Number = 19778
Date of Application = 16,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = PRINZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;20MG
----
Product id = 26693
Application Number = 19778
Date of Application = 18,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = PRINZIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 29771
Application Number = 19888
Date of Application = 20,, Sep, 1990
RX/OTC/DISCN = RX
Tradename = ZESTORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 12.5MG;20MG
----
Product id = 29772
Application Number = 19888
Date of Application = 20,, Jul, 1989
RX/OTC/DISCN = RX
Tradename = ZESTORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;20MG
----
Product id = 29770
Application Number = 19888
Date of Application = 18,, Nov, 1993
RX/OTC/DISCN = RX
Tradename = ZESTORETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 24042
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 24043
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 24045
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 20MG;25MG
----
Product id = 24044
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 17202
Application Number = 20125
Date of Application = 28,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = ACCURETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 17203
Application Number = 20125
Date of Application = 28,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = ACCURETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 17204
Application Number = 20125
Date of Application = 28,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = ACCURETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 25MG;EQ 20MG BASE
----
Product id = 29781
Application Number = 20186
Date of Application = 26,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ZIAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 29782
Application Number = 20186
Date of Application = 26,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ZIAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 10MG;6.25MG
----
Product id = 29780
Application Number = 20186
Date of Application = 26,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ZIAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 25072
Application Number = 20286
Date of Application = 30,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = MONOPRIL-HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 25071
Application Number = 20286
Date of Application = 30,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = MONOPRIL-HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 22684
Application Number = 20387
Date of Application = 28,, Apr, 1995
RX/OTC/DISCN = RX
Tradename = HYZAAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 22686
Application Number = 20387
Date of Application = 10,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = HYZAAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;100MG
----
Product id = 22685
Application Number = 20387
Date of Application = 20,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = HYZAAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 2504
Application Number = 20504
Date of Application = 27,, Dec, 1996
RX/OTC/DISCN = RX
Tradename = MICROZIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ACTAVIS LABS UT INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 12.5MG
----
Product id = 29287
Application Number = 20729
Date of Application = 27,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = UNIRETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;7.5MG
----
Product id = 29289
Application Number = 20729
Date of Application = 27,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = UNIRETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;15MG
----
Product id = 29288
Application Number = 20729
Date of Application = 14,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = UNIRETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = UCB INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;15MG
----
Product id = 18218
Application Number = 20758
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = AVALIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;75MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18219
Application Number = 20758
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = AVALIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 12.5MG;150MG
----
Product id = 18220
Application Number = 20758
Date of Application = 31,, Aug, 1998
RX/OTC/DISCN = RX
Tradename = AVALIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 12.5MG;300MG
----
Product id = 18221
Application Number = 20758
Date of Application = 15,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = AVALIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 004
Tecode =
Rld = No
Strength = 25MG;300MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 20497
Application Number = 20818
Date of Application = 6,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = DIOVAN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 20498
Application Number = 20818
Date of Application = 6,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = DIOVAN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 20500
Application Number = 20818
Date of Application = 17,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = DIOVAN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 20499
Application Number = 20818
Date of Application = 28,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = DIOVAN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 20501
Application Number = 20818
Date of Application = 28,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = DIOVAN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 25MG;320MG
----
Product id = 18091
Application Number = 21093
Date of Application = 5,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = ATACAND HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18092
Application Number = 21093
Date of Application = 5,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = ATACAND HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18093
Application Number = 21093
Date of Application = 16,, May, 2008
RX/OTC/DISCN = RX
Tradename = ATACAND HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 32MG;25MG
----
Product id = 24910
Application Number = 21162
Date of Application = 17,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = MICARDIS HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 24911
Application Number = 21162
Date of Application = 17,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = MICARDIS HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 24912
Application Number = 21162
Date of Application = 19,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = MICARDIS HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 25MG;80MG
----
Product id = 28604
Application Number = 21268
Date of Application = 1,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = TEVETEN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG;12.5MG
----
Product id = 28605
Application Number = 21268
Date of Application = 1,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = TEVETEN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode =
Rld = Yes
Strength = 600MG;25MG
----
Product id = 18418
Application Number = 21532
Date of Application = 5,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = BENICAR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;20MG
----
Product id = 18419
Application Number = 21532
Date of Application = 5,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = BENICAR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 003
Tecode =
Rld = No
Strength = 12.5MG;40MG
----
Product id = 18420
Application Number = 21532
Date of Application = 5,, Jun, 2003
RX/OTC/DISCN = RX
Tradename = BENICAR HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 005
Tecode =
Rld = Yes
Strength = 25MG;40MG
----
Product id = 15901
Application Number = 21956
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = DUTOPROL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = COVIS PHARMA SARL
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;EQ 25MG TARTRATE
----
Product id = 15902
Application Number = 21956
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = DUTOPROL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = COVIS PHARMA SARL
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;EQ 50MG TARTRATE
----
Product id = 15903
Application Number = 21956
Date of Application = 28,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = DUTOPROL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = COVIS PHARMA SARL
ProductNo = 003
Tecode =
Rld = Yes
Strength = 12.5MG;EQ 100MG TARTRATE
----
Product id = 28497
Application Number = 22107
Date of Application = 18,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = TEKTURNA HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE;12.5MG
----
Product id = 28498
Application Number = 22107
Date of Application = 18,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = TEKTURNA HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 150MG BASE;25MG
----
Product id = 28499
Application Number = 22107
Date of Application = 18,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = TEKTURNA HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = Yes
Strength = EQ 300MG BASE;12.5MG
----
Product id = 28500
Application Number = 22107
Date of Application = 18,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = TEKTURNA HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode =
Rld = Yes
Strength = EQ 300MG BASE;25MG
----
Product id = 21132
Application Number = 22314
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = EXFORGE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;12.5MG;160MG
----
Product id = 21133
Application Number = 22314
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = EXFORGE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;25MG;160MG
----
Product id = 21134
Application Number = 22314
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = EXFORGE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;12.5MG;160MG
----
Product id = 21135
Application Number = 22314
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = EXFORGE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 10MG;25MG;160MG
----
Product id = 21136
Application Number = 22314
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = EXFORGE HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 10MG;25MG;320MG
----
Product id = 22358
Application Number = 40412
Date of Application = 29,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22359
Application Number = 40412
Date of Application = 29,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22297
Application Number = 40702
Date of Application = 16,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22298
Application Number = 40702
Date of Application = 16,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EXCELLIUM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22281
Application Number = 40707
Date of Application = 27,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 22329
Application Number = 40735
Date of Application = 23,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22330
Application Number = 40735
Date of Application = 23,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22328
Application Number = 40770
Date of Application = 23,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 22286
Application Number = 40774
Date of Application = 3,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22287
Application Number = 40774
Date of Application = 3,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22290
Application Number = 40780
Date of Application = 20,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22291
Application Number = 40780
Date of Application = 20,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22310
Application Number = 40807
Date of Application = 20,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 22311
Application Number = 40807
Date of Application = 20,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22312
Application Number = 40807
Date of Application = 20,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22317
Application Number = 40809
Date of Application = 4,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JUBILANT CADISTA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22318
Application Number = 40809
Date of Application = 4,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = JUBILANT CADISTA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22343
Application Number = 40810
Date of Application = 27,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22344
Application Number = 40810
Date of Application = 27,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22342
Application Number = 40857
Date of Application = 30,, May, 2008
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 22352
Application Number = 40907
Date of Application = 15,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22353
Application Number = 40907
Date of Application = 15,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24693
Application Number = 70182
Date of Application = 15,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24695
Application Number = 70183
Date of Application = 15,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24680
Application Number = 70265
Date of Application = 23,, Jan, 1986
RX/OTC/DISCN = RX
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 25MG;250MG
----
Product id = 24679
Application Number = 70265
Date of Application = 23,, Jan, 1986
RX/OTC/DISCN = RX
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 27021
Application Number = 70301
Date of Application = 18,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27023
Application Number = 70305
Date of Application = 18,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 22274
Application Number = 70347
Date of Application = 25,, Dec, 1990
RX/OTC/DISCN = DISCN
Tradename = HYDRO-RIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG ANHYDROUS;50MG
----
Product id = 24703
Application Number = 70365
Date of Application = 19,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24706
Application Number = 70366
Date of Application = 16,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24709
Application Number = 70367
Date of Application = 19,, Mar, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24712
Application Number = 70368
Date of Application = 16,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 24697
Application Number = 70543
Date of Application = 15,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24698
Application Number = 70544
Date of Application = 15,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 24682
Application Number = 70612
Date of Application = 2,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24683
Application Number = 70613
Date of Application = 2,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24684
Application Number = 70614
Date of Application = 2,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 24681
Application Number = 70616
Date of Application = 2,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24690
Application Number = 70688
Date of Application = 24,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24692
Application Number = 70689
Date of Application = 24,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 27009
Application Number = 70704
Date of Application = 1,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27010
Application Number = 70705
Date of Application = 1,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 17607
Application Number = 70795
Date of Application = 17,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = AMILORIDE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG ANHYDROUS;50MG
----
Product id = 24694
Application Number = 70829
Date of Application = 9,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24696
Application Number = 70830
Date of Application = 9,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 27007
Application Number = 70851
Date of Application = 15,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27008
Application Number = 70852
Date of Application = 15,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 24689
Application Number = 70853
Date of Application = 8,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24691
Application Number = 70854
Date of Application = 8,, Oct, 1986
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 27014
Application Number = 70947
Date of Application = 1,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG;80MG
----
Product id = 27013
Application Number = 70947
Date of Application = 4,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;40MG
----
Product id = 24704
Application Number = 70958
Date of Application = 6,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24707
Application Number = 70959
Date of Application = 19,, Jan, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24713
Application Number = 70960
Date of Application = 6,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 27017
Application Number = 71060
Date of Application = 26,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27018
Application Number = 71061
Date of Application = 26,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 24710
Application Number = 71069
Date of Application = 19,, Jan, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 17604
Application Number = 71111
Date of Application = 10,, May, 1988
RX/OTC/DISCN = RX
Tradename = AMILORIDE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG ANHYDROUS;50MG
----
Product id = 27005
Application Number = 71126
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE & HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27006
Application Number = 71127
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE & HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 29126
Application Number = 71251
Date of Application = 17,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;75MG
----
Product id = 29125
Application Number = 71251
Date of Application = 5,, May, 1998
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 24675
Application Number = 71458
Date of Application = 8,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24676
Application Number = 71459
Date of Application = 8,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24677
Application Number = 71460
Date of Application = 8,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24678
Application Number = 71461
Date of Application = 8,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 27022
Application Number = 71498
Date of Application = 18,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27024
Application Number = 71501
Date of Application = 18,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 27011
Application Number = 71552
Date of Application = 1,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27012
Application Number = 71553
Date of Application = 1,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 3650
Application Number = 71737
Date of Application = 12,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = VITARINE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;50MG
----
Product id = 27019
Application Number = 71771
Date of Application = 26,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;40MG
----
Product id = 27020
Application Number = 71772
Date of Application = 26,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;80MG
----
Product id = 24699
Application Number = 71819
Date of Application = 8,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24700
Application Number = 71820
Date of Application = 8,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24701
Application Number = 71821
Date of Application = 8,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24702
Application Number = 71822
Date of Application = 8,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 29133
Application Number = 71851
Date of Application = 30,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;75MG
----
Product id = 24685
Application Number = 71897
Date of Application = 23,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24686
Application Number = 71898
Date of Application = 23,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24687
Application Number = 71899
Date of Application = 23,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24688
Application Number = 71900
Date of Application = 23,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 24705
Application Number = 71920
Date of Application = 29,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24708
Application Number = 71921
Date of Application = 29,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24711
Application Number = 71922
Date of Application = 29,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24714
Application Number = 71923
Date of Application = 29,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 29134
Application Number = 71969
Date of Application = 17,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;75MG
----
Product id = 29129
Application Number = 71980
Date of Application = 17,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = QUANTUM PHARMICS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;75MG
----
Product id = 29131
Application Number = 72011
Date of Application = 17,, Jun, 1988
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;75MG
----
Product id = 29124
Application Number = 72022
Date of Application = 17,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;75MG
----
Product id = 27015
Application Number = 72042
Date of Application = 14,, Mar, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;40MG
----
Product id = 27016
Application Number = 72043
Date of Application = 14,, Mar, 1988
RX/OTC/DISCN = RX
Tradename = PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 24671
Application Number = 72507
Date of Application = 2,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 15MG;250MG
----
Product id = 24672
Application Number = 72508
Date of Application = 2,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;250MG
----
Product id = 24673
Application Number = 72509
Date of Application = 2,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG;500MG
----
Product id = 24674
Application Number = 72510
Date of Application = 2,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = METHYLDOPA AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;500MG
----
Product id = 3649
Application Number = 73191
Date of Application = 31,, Jul, 1991
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 25MG;50MG
----
Product id = 17605
Application Number = 73209
Date of Application = 31,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = AMILORIDE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 5MG ANHYDROUS;50MG
----
Product id = 29130
Application Number = 73281
Date of Application = 30,, Apr, 1992
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 17608
Application Number = 73334
Date of Application = 19,, Jul, 1991
RX/OTC/DISCN = DISCN
Tradename = AMILORIDE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG ANHYDROUS;50MG
----
Product id = 17606
Application Number = 73357
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = AMILORIDE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG ANHYDROUS;50MG
----
Product id = 29132
Application Number = 73449
Date of Application = 23,, Sep, 1993
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 29128
Application Number = 73467
Date of Application = 31,, Jan, 1996
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;75MG
----
Product id = 29127
Application Number = 74026
Date of Application = 26,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 3644
Application Number = 74259
Date of Application = 30,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 3646
Application Number = 74701
Date of Application = 7,, Jun, 1996
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 18946
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 18947
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 18949
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 18948
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG;15MG
----
Product id = 3648
Application Number = 74821
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 18942
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;15MG
----
Product id = 18943
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;25MG
----
Product id = 18945
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18944
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 50MG;15MG
----
Product id = 18950
Application Number = 74832
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 3647
Application Number = 74857
Date of Application = 9,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;37.5MG
----
Product id = 18938
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;15MG
----
Product id = 18939
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;25MG
----
Product id = 18941
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18940
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 50MG;15MG
----
Product id = 3642
Application Number = 74970
Date of Application = 6,, Jan, 1998
RX/OTC/DISCN = DISCN
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;37.5MG
----
Product id = 3643
Application Number = 75052
Date of Application = 18,, Jun, 1999
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 18934
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 18935
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 18937
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 18936
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG;15MG
----
Product id = 18602
Application Number = 75469
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18603
Application Number = 75469
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18604
Application Number = 75469
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18590
Application Number = 75527
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18594
Application Number = 75527
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18592
Application Number = 75527
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18591
Application Number = 75579
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18593
Application Number = 75579
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18595
Application Number = 75579
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;6.25MG
----
Product id = 20862
Application Number = 75624
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;12.5MG
----
Product id = 20863
Application Number = 75624
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;25MG
----
Product id = 18584
Application Number = 75632
Date of Application = 27,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18585
Application Number = 75632
Date of Application = 27,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18586
Application Number = 75632
Date of Application = 27,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 2161
Application Number = 75640
Date of Application = 28,, Jan, 2000
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18582
Application Number = 75642
Date of Application = 27,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18581
Application Number = 75642
Date of Application = 27,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18583
Application Number = 75642
Date of Application = 27,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18578
Application Number = 75672
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18579
Application Number = 75672
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18580
Application Number = 75672
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18596
Application Number = 75686
Date of Application = 19,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18597
Application Number = 75686
Date of Application = 19,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18598
Application Number = 75686
Date of Application = 19,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 20868
Application Number = 75727
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;12.5MG
----
Product id = 20869
Application Number = 75727
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;25MG
----
Product id = 20860
Application Number = 75736
Date of Application = 25,, Mar, 2003
RX/OTC/DISCN = DISCN
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;12.5MG
----
Product id = 20861
Application Number = 75736
Date of Application = 25,, Mar, 2003
RX/OTC/DISCN = DISCN
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;25MG
----
Product id = 18587
Application Number = 75768
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18588
Application Number = 75768
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18589
Application Number = 75768
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;6.25MG
----
Product id = 23803
Application Number = 75776
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23804
Application Number = 75776
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23805
Application Number = 75776
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 20866
Application Number = 75788
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;12.5MG
----
Product id = 20867
Application Number = 75788
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;25MG
----
Product id = 23824
Application Number = 75869
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23825
Application Number = 75869
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23826
Application Number = 75869
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;20MG
----
Product id = 2164
Application Number = 75907
Date of Application = 17,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 20858
Application Number = 75909
Date of Application = 15,, Oct, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;12.5MG
----
Product id = 20859
Application Number = 75909
Date of Application = 15,, Oct, 2001
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;25MG
----
Product id = 23818
Application Number = 75926
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23820
Application Number = 75926
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23822
Application Number = 75926
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = DISCN
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;20MG
----
Product id = 23815
Application Number = 76007
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23816
Application Number = 76007
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23817
Application Number = 76007
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23809
Application Number = 76113
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23810
Application Number = 76113
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23811
Application Number = 76113
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 20864
Application Number = 76116
Date of Application = 19,, Sep, 2001
RX/OTC/DISCN = DISCN
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;12.5MG
----
Product id = 20865
Application Number = 76116
Date of Application = 19,, Sep, 2001
RX/OTC/DISCN = DISCN
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;25MG
----
Product id = 23828
Application Number = 76194
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23829
Application Number = 76194
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23827
Application Number = 76194
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23812
Application Number = 76230
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23813
Application Number = 76230
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23814
Application Number = 76230
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23819
Application Number = 76262
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23821
Application Number = 76262
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23823
Application Number = 76262
Date of Application = 1,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 23800
Application Number = 76265
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23801
Application Number = 76265
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23802
Application Number = 76265
Date of Application = 8,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 18386
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18387
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18388
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18389
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 18394
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18395
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18396
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18397
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 27240
Application Number = 76374
Date of Application = 31,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = QUINARETIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GAVIS PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27241
Application Number = 76374
Date of Application = 31,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = QUINARETIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GAVIS PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27242
Application Number = 76374
Date of Application = 31,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = QUINARETIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GAVIS PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 20856
Application Number = 76486
Date of Application = 27,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;12.5MG
----
Product id = 20857
Application Number = 76486
Date of Application = 27,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = ENALAPRIL MALEATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;25MG
----
Product id = 21587
Application Number = 76608
Date of Application = 3,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21588
Application Number = 76608
Date of Application = 3,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18402
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18403
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18404
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18405
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode =
Rld = No
Strength = 20MG;25MG
----
Product id = 18410
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18411
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18412
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18413
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 23794
Application Number = 76674
Date of Application = 5,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23795
Application Number = 76674
Date of Application = 5,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23796
Application Number = 76674
Date of Application = 5,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 18398
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18399
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18400
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18401
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 21597
Application Number = 76739
Date of Application = 17,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21598
Application Number = 76739
Date of Application = 17,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 24862
Application Number = 76792
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 24863
Application Number = 76792
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24864
Application Number = 76792
Date of Application = 20,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;100MG
----
Product id = 21601
Application Number = 76945
Date of Application = 5,, Jul, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21602
Application Number = 76945
Date of Application = 5,, Jul, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21599
Application Number = 76961
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21600
Application Number = 76961
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 25059
Application Number = 76980
Date of Application = 7,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;7.5MG
----
Product id = 25061
Application Number = 76980
Date of Application = 7,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;15MG
----
Product id = 25060
Application Number = 76980
Date of Application = 7,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;15MG
----
Product id = 2158
Application Number = 77005
Date of Application = 13,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 27234
Application Number = 77093
Date of Application = 28,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27235
Application Number = 77093
Date of Application = 28,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27236
Application Number = 77093
Date of Application = 28,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 21603
Application Number = 77144
Date of Application = 16,, Aug, 2005
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21604
Application Number = 77144
Date of Application = 16,, Aug, 2005
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 24026
Application Number = 77157
Date of Application = 6,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24027
Application Number = 77157
Date of Application = 6,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24028
Application Number = 77157
Date of Application = 6,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 23020
Application Number = 77369
Date of Application = 30,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23021
Application Number = 77369
Date of Application = 30,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 23022
Application Number = 77369
Date of Application = 30,, Mar, 2012
RX/OTC/DISCN = DISCN
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;300MG
----
Product id = 23018
Application Number = 77446
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23019
Application Number = 77446
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 18406
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18407
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18408
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18409
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 23797
Application Number = 77606
Date of Application = 14,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23798
Application Number = 77606
Date of Application = 14,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23799
Application Number = 77606
Date of Application = 14,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 21595
Application Number = 77705
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21596
Application Number = 77705
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 24021
Application Number = 77732
Date of Application = 6,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24020
Application Number = 77732
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24022
Application Number = 77732
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 2156
Application Number = 77885
Date of Application = 26,, Nov, 2007
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 23806
Application Number = 77912
Date of Application = 27,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 23807
Application Number = 77912
Date of Application = 27,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 23808
Application Number = 77912
Date of Application = 27,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = LISINOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 24023
Application Number = 77948
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24025
Application Number = 77948
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24024
Application Number = 77948
Date of Application = 19,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 23012
Application Number = 77969
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23013
Application Number = 77969
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 29433
Application Number = 78020
Date of Application = 21,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 29434
Application Number = 78020
Date of Application = 21,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 29436
Application Number = 78020
Date of Application = 21,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 29435
Application Number = 78020
Date of Application = 21,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 29437
Application Number = 78020
Date of Application = 21,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 25MG;320MG
----
Product id = 2155
Application Number = 78164
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 27237
Application Number = 78211
Date of Application = 4,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27238
Application Number = 78211
Date of Application = 4,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27239
Application Number = 78211
Date of Application = 4,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 24011
Application Number = 78245
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24012
Application Number = 78245
Date of Application = 21,, May, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24013
Application Number = 78245
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24035
Application Number = 78385
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24036
Application Number = 78385
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 2154
Application Number = 78389
Date of Application = 16,, May, 2008
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 2159
Application Number = 78391
Date of Application = 11,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = JUBILANT CADISTA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 27228
Application Number = 78450
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27229
Application Number = 78450
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27230
Application Number = 78450
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 18390
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18391
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18392
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18393
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 29425
Application Number = 78946
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 29427
Application Number = 78946
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;320MG
----
Product id = 29423
Application Number = 78946
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 29424
Application Number = 78946
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 29426
Application Number = 78946
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 21591
Application Number = 79025
Date of Application = 17,, Sep, 2010
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EMCURE PHARMS INDIA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21592
Application Number = 79025
Date of Application = 17,, Sep, 2010
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EMCURE PHARMS INDIA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 20MG;12.5MG
----
Product id = 18599
Application Number = 79106
Date of Application = 28,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18600
Application Number = 79106
Date of Application = 28,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18601
Application Number = 79106
Date of Application = 28,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;6.25MG
----
Product id = 2157
Application Number = 79237
Date of Application = 2,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 21589
Application Number = 79245
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21590
Application Number = 79245
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 22362
Application Number = 81189
Date of Application = 24,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22370
Application Number = 81190
Date of Application = 24,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22313
Application Number = 83177
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22314
Application Number = 83177
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 50MG
----
Product id = 22365
Application Number = 83232
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22366
Application Number = 83456
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22363
Application Number = 83458
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22285
Application Number = 83554
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALRA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22380
Application Number = 83568
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.1MG
----
Product id = 22379
Application Number = 83571
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22378
Application Number = 83572
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.1MG
----
Product id = 22381
Application Number = 83573
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22305
Application Number = 83607
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22388
Application Number = 83666
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22391
Application Number = 83770
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE AND HYDRALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22375
Application Number = 83809
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22374
Application Number = 83809
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22575
Application Number = 83877
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROSERPINE PLUS (R-H-H)
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22272
Application Number = 83891
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRO-D
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22335
Application Number = 83899
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 29784
Application Number = 83925
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22351
Application Number = 83965
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TG UNITED LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22323
Application Number = 83972
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22324
Application Number = 83972
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22325
Application Number = 83972
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22304
Application Number = 84029
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22299
Application Number = 84135
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HEATHER
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22267
Application Number = 84291
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE, HYDROCHLOROTHIAZIDE AND RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22320
Application Number = 84324
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22319
Application Number = 84325
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22385
Application Number = 84466
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22389
Application Number = 84467
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22333
Application Number = 84536
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 27500
Application Number = 84579
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27499
Application Number = 84580
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22384
Application Number = 84603
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22315
Application Number = 84658
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22273
Application Number = 84714
Date of Application = 29,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRO-RESERP
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABC HOLDING
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 1096
Application Number = 84735
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = APRESAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 22292
Application Number = 84771
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22307
Application Number = 84776
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22309
Application Number = 84776
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 1097
Application Number = 84810
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = APRESAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;50MG
----
Product id = 1098
Application Number = 84811
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = APRESAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG;50MG
----
Product id = 22275
Application Number = 84827
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRO-SERP "25"
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22270
Application Number = 84876
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRAP-ES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22303
Application Number = 84878
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22302
Application Number = 84878
Date of Application = 12,, Jul, 2006
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22326
Application Number = 84880
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18794
Application Number = 84897
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CAM-AP-ES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TG UNITED LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22373
Application Number = 84899
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22337
Application Number = 84912
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22332
Application Number = 85004
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22334
Application Number = 85005
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22316
Application Number = 85022
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22282
Application Number = 85054
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22308
Application Number = 85067
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22306
Application Number = 85098
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IMPAX LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22371
Application Number = 85099
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22327
Application Number = 85112
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22296
Application Number = 85152
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ELKINS SINN
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22301
Application Number = 85182
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22300
Application Number = 85182
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22283
Application Number = 85208
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22276
Application Number = 85213
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRO-SERP "50"
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22338
Application Number = 85219
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22364
Application Number = 85232
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22367
Application Number = 85233
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22386
Application Number = 85317
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22341
Application Number = 85323
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22049
Application Number = 85338
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = H.R.-50
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22376
Application Number = 85347
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WHITEWORTH TOWN PLSN
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22377
Application Number = 85373
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ HYDRALAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 22383
Application Number = 85420
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHARMERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22382
Application Number = 85421
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PHARMERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 2148
Application Number = 85440
Date of Application = 4,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG;50MG
----
Product id = 2147
Application Number = 85446
Date of Application = 4,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;50MG
----
Product id = 2146
Application Number = 85457
Date of Application = 4,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 27507
Application Number = 85549
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22277
Application Number = 85672
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABC HOLDING
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22350
Application Number = 85683
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TG UNITED LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22269
Application Number = 85771
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE, HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22171
Application Number = 85827
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE AND HYDROCHLORTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 29285
Application Number = 85893
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPRES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 28225
Application Number = 85974
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 28226
Application Number = 86026
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 22368
Application Number = 86087
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22321
Application Number = 86192
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MAST MM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22322
Application Number = 86192
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MAST MM
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 29286
Application Number = 86298
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = UNIPRES
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22387
Application Number = 86330
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;0.125MG
----
Product id = 22390
Application Number = 86331
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE W/ RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 22284
Application Number = 86369
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALRA
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22271
Application Number = 86504
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRO-D
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HALSEY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 28213
Application Number = 86513
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;25MG
----
Product id = 22369
Application Number = 86594
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22331
Application Number = 86597
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PVT FORM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28215
Application Number = 86881
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 22372
Application Number = 87002
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 28218
Application Number = 87004
Date of Application = 24,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 22293
Application Number = 87059
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22295
Application Number = 87060
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22294
Application Number = 87068
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 22268
Application Number = 87085
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE-HYDROCHLOROTHIAZIDE-RESERPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27870
Application Number = 87210
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SER-A-GEN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 2142
Application Number = 87213
Date of Application = 8,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;50MG
----
Product id = 28211
Application Number = 87267
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 28217
Application Number = 87398
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 28219
Application Number = 87511
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 22288
Application Number = 87539
Date of Application = 3,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ASCOT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22289
Application Number = 87540
Date of Application = 3,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ASCOT
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 28222
Application Number = 87553
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UPSHER SMITH
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 27508
Application Number = 87556
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22336
Application Number = 87565
Date of Application = 9,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22360
Application Number = 87586
Date of Application = 3,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22361
Application Number = 87587
Date of Application = 3,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 2141
Application Number = 87608
Date of Application = 8,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 2143
Application Number = 87609
Date of Application = 8,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG;50MG
----
Product id = 22357
Application Number = 87610
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22356
Application Number = 87638
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 28223
Application Number = 87651
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 28224
Application Number = 87655
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 27509
Application Number = 87709
Date of Application = 13,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDROCHLOROTHIAZIDE, AND HYDRALAZINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 22355
Application Number = 87752
Date of Application = 19,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22354
Application Number = 87827
Date of Application = 19,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 28220
Application Number = 87948
Date of Application = 22,, Feb, 1983
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 28214
Application Number = 87999
Date of Application = 6,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 28210
Application Number = 88025
Date of Application = 23,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ASCOT
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 28221
Application Number = 88054
Date of Application = 18,, Aug, 1983
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE W/ HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 27502
Application Number = 88189
Date of Application = 10,, May, 1984
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE-50
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 27501
Application Number = 88200
Date of Application = 31,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = RESERPINE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;0.125MG
----
Product id = 2150
Application Number = 88356
Date of Application = 10,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE W/ HYDROCHLOROTHIAZIDE 25/25
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 2151
Application Number = 88357
Date of Application = 10,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE W/ HYDROCHLOROTHIAZIDE 50/50
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;50MG
----
Product id = 2149
Application Number = 88358
Date of Application = 10,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE W/ HYDROCHLOROTHIAZIDE 100/50
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG;50MG
----
Product id = 27506
Application Number = 88376
Date of Application = 28,, Oct, 1983
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 27505
Application Number = 88570
Date of Application = 10,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = RESERPINE, HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG;0.1MG
----
Product id = 13835
Application Number = 88587
Date of Application = 2,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/5ML
----
Product id = 13836
Application Number = 88588
Date of Application = 2,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE INTENSOL
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 22345
Application Number = 88827
Date of Application = 28,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 22346
Application Number = 88828
Date of Application = 28,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22347
Application Number = 88829
Date of Application = 28,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 22349
Application Number = 88923
Date of Application = 7,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 22348
Application Number = 88924
Date of Application = 7,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 2139
Application Number = 88946
Date of Application = 21,, Oct, 1985
RX/OTC/DISCN = RX
Tradename = HYDRA-ZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG;50MG
----
Product id = 2138
Application Number = 88957
Date of Application = 21,, Oct, 1985
RX/OTC/DISCN = RX
Tradename = HYDRA-ZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 2140
Application Number = 88961
Date of Application = 21,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = HYDRA-ZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG;50MG
----
Product id = 28216
Application Number = 89137
Date of Application = 26,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 2144
Application Number = 89200
Date of Application = 9,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 2145
Application Number = 89201
Date of Application = 9,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = HYDRALAZINE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SUPERPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;50MG
----
Product id = 28212
Application Number = 89534
Date of Application = 2,, Jul, 1987
RX/OTC/DISCN = RX
Tradename = SPIRONOLACTONE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;25MG
----
Product id = 13834
Application Number = 89661
Date of Application = 20,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/5ML
----
Product id = 25056
Application Number = 90096
Date of Application = 25,, Sep, 2008
RX/OTC/DISCN = DISCN
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;7.5MG
----
Product id = 25057
Application Number = 90096
Date of Application = 25,, Sep, 2008
RX/OTC/DISCN = DISCN
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;15MG
----
Product id = 25058
Application Number = 90096
Date of Application = 25,, Sep, 2008
RX/OTC/DISCN = DISCN
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 23999
Application Number = 90150
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24000
Application Number = 90150
Date of Application = 11,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24001
Application Number = 90150
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 21593
Application Number = 90228
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21594
Application Number = 90228
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 23016
Application Number = 90351
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23017
Application Number = 90351
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 2163
Application Number = 90510
Date of Application = 19,, Jan, 2010
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = UNICHEM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 24029
Application Number = 90528
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24031
Application Number = 90528
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24030
Application Number = 90528
Date of Application = 6,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 2162
Application Number = 90651
Date of Application = 7,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 24865
Application Number = 90654
Date of Application = 19,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 24866
Application Number = 90654
Date of Application = 19,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24867
Application Number = 90654
Date of Application = 19,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;100MG
----
Product id = 18819
Application Number = 90704
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18820
Application Number = 90704
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18821
Application Number = 90704
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----
Product id = 25053
Application Number = 90718
Date of Application = 17,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;7.5MG
----
Product id = 25054
Application Number = 90718
Date of Application = 17,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;15MG
----
Product id = 25055
Application Number = 90718
Date of Application = 17,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;15MG
----
Product id = 28538
Application Number = 91351
Date of Application = 7,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28539
Application Number = 91351
Date of Application = 7,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28540
Application Number = 91351
Date of Application = 7,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 23000
Application Number = 91370
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23001
Application Number = 91370
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 29438
Application Number = 91519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 29439
Application Number = 91519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 29440
Application Number = 91519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 29441
Application Number = 91519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 29442
Application Number = 91519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 25MG;320MG
----
Product id = 27225
Application Number = 91524
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27226
Application Number = 91524
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27227
Application Number = 91524
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 23023
Application Number = 91539
Date of Application = 22,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23024
Application Number = 91539
Date of Application = 22,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 23996
Application Number = 91617
Date of Application = 17,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 23997
Application Number = 91617
Date of Application = 17,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 23998
Application Number = 91617
Date of Application = 17,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24002
Application Number = 91629
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24003
Application Number = 91629
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24004
Application Number = 91629
Date of Application = 6,, Jan, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 28541
Application Number = 91648
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28542
Application Number = 91648
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28543
Application Number = 91648
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 24017
Application Number = 91652
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24018
Application Number = 91652
Date of Application = 6,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24019
Application Number = 91652
Date of Application = 6,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 2160
Application Number = 91662
Date of Application = 27,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANNETT HOLDINGS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 17992
Application Number = 200045
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = AMTURNIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE;EQ 5MG BASE;12.5MG
----
Product id = 17993
Application Number = 200045
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = AMTURNIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 300MG BASE;EQ 5MG BASE;12.5MG
----
Product id = 17994
Application Number = 200045
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = AMTURNIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 300MG BASE;EQ 5MG BASE;25MG
----
Product id = 17995
Application Number = 200045
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = AMTURNIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 300MG BASE;EQ 10MG BASE;12.5MG
----
Product id = 17996
Application Number = 200045
Date of Application = 21,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = AMTURNIDE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode =
Rld = Yes
Strength = EQ 300MG BASE;EQ 10MG BASE;25MG
----
Product id = 29146
Application Number = 200175
Date of Application = 23,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = TRIBENZOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE;12.5MG;20MG
----
Product id = 29147
Application Number = 200175
Date of Application = 23,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = TRIBENZOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 5MG BASE;12.5MG;40MG
----
Product id = 29148
Application Number = 200175
Date of Application = 23,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = TRIBENZOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 5MG BASE;25MG;40MG
----
Product id = 29149
Application Number = 200175
Date of Application = 23,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = TRIBENZOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 10MG BASE;12.5MG;40MG
----
Product id = 29150
Application Number = 200175
Date of Application = 23,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = TRIBENZOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = DAIICHI SANKYO
ProductNo = 005
Tecode =
Rld = Yes
Strength = EQ 10MG BASE;25MG;40MG
----
Product id = 24032
Application Number = 200180
Date of Application = 12,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24033
Application Number = 200180
Date of Application = 12,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24034
Application Number = 200180
Date of Application = 12,, Jan, 2011
RX/OTC/DISCN = DISCN
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG;100MG
----
Product id = 17939
Application Number = 200435
Date of Application = 25,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE,VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;12.5MG;160MG
----
Product id = 17940
Application Number = 200435
Date of Application = 25,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE,VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;25MG;160MG
----
Product id = 17942
Application Number = 200435
Date of Application = 25,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE,VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;25MG;160MG
----
Product id = 17943
Application Number = 200435
Date of Application = 25,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE,VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 10MG;25MG;320MG
----
Product id = 17941
Application Number = 200435
Date of Application = 25,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE,VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 10MG;12.5MG;160MG
----
Product id = 2153
Application Number = 200645
Date of Application = 30,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 28544
Application Number = 201192
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28545
Application Number = 201192
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28546
Application Number = 201192
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 27231
Application Number = 201356
Date of Application = 20,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 27232
Application Number = 201356
Date of Application = 20,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 27233
Application Number = 201356
Date of Application = 20,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 3645
Application Number = 201407
Date of Application = 9,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = TRIAMTERENE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANNETT HOLDINGS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;37.5MG
----
Product id = 23002
Application Number = 201505
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23003
Application Number = 201505
Date of Application = 15,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 23008
Application Number = 201524
Date of Application = 27,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23009
Application Number = 201524
Date of Application = 27,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 29408
Application Number = 201662
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 29409
Application Number = 201662
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 29410
Application Number = 201662
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 29411
Application Number = 201662
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 29412
Application Number = 201662
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 25MG;320MG
----
Product id = 24008
Application Number = 201682
Date of Application = 1,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24009
Application Number = 201682
Date of Application = 1,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24010
Application Number = 201682
Date of Application = 1,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24005
Application Number = 201845
Date of Application = 18,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24006
Application Number = 201845
Date of Application = 18,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24007
Application Number = 201845
Date of Application = 18,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 25062
Application Number = 202150
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHOLROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;7.5MG
----
Product id = 25063
Application Number = 202150
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHOLROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;15MG
----
Product id = 25064
Application Number = 202150
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = MOEXIPRIL HYDROCHLORIDE AND HYDROCHOLROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HERITAGE PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;15MG
----
Product id = 24014
Application Number = 202289
Date of Application = 9,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;50MG
----
Product id = 24015
Application Number = 202289
Date of Application = 9,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;100MG
----
Product id = 24016
Application Number = 202289
Date of Application = 9,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = LOSARTAN POTASSIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 23010
Application Number = 202414
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23011
Application Number = 202414
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 29418
Application Number = 202519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 29419
Application Number = 202519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 29420
Application Number = 202519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 29421
Application Number = 202519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 29422
Application Number = 202519
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 25MG;320MG
----
Product id = 22278
Application Number = 202556
Date of Application = 24,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 22279
Application Number = 202556
Date of Application = 24,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22280
Application Number = 202556
Date of Application = 24,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 24859
Application Number = 202870
Date of Application = 6,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;50MG
----
Product id = 24860
Application Number = 202870
Date of Application = 6,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;100MG
----
Product id = 24861
Application Number = 202870
Date of Application = 6,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;100MG
----
Product id = 18810
Application Number = 202884
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18811
Application Number = 202884
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18812
Application Number = 202884
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----
Product id = 18813
Application Number = 202965
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18814
Application Number = 202965
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18815
Application Number = 202965
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----
Product id = 28535
Application Number = 203010
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28536
Application Number = 203010
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28537
Application Number = 203010
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 22339
Application Number = 203018
Date of Application = 23,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCIEGEN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 22340
Application Number = 203018
Date of Application = 23,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCIEGEN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 29413
Application Number = 203026
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 29414
Application Number = 203026
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 29415
Application Number = 203026
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 29416
Application Number = 203026
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 29417
Application Number = 203026
Date of Application = 21,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 25MG;320MG
----
Product id = 23014
Application Number = 203072
Date of Application = 9,, May, 2014
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23015
Application Number = 203072
Date of Application = 9,, May, 2014
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 29428
Application Number = 203145
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 29429
Application Number = 203145
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;160MG
----
Product id = 29430
Application Number = 203145
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 12.5MG;320MG
----
Product id = 29431
Application Number = 203145
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 25MG;160MG
----
Product id = 29432
Application Number = 203145
Date of Application = 19,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = VALSARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 25MG;320MG
----
Product id = 23006
Application Number = 203500
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23007
Application Number = 203500
Date of Application = 27,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 23004
Application Number = 203630
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;150MG
----
Product id = 23005
Application Number = 203630
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = IRBESARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;300MG
----
Product id = 18816
Application Number = 204100
Date of Application = 27,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18817
Application Number = 204100
Date of Application = 27,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18818
Application Number = 204100
Date of Application = 27,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----